3 1 Separation Of Group I Cations Chemistry Libretexts

3 1 Separation Of Group I Cations Chemistry Libretexts The precipitate is resuspended in pure water by stirring with a clean glass rod, centrifuged, and decanted again to wash out any residual impurities. the washed precipitate is used to separate and confirm the group 1 cations and the supernatant is saved for analysis of group 2, 3, 4, and 5 cations. pb2 (aq) 2cl − (aq) − ⇀ ↽ − pbcl. 3.1: separation of group i cations group i cations are separated based on the solubility rule that states "salts of chloride, bromide, and iodide are soluble, except when the cation is lead(ii), mercury(i), or silver(i). hydrochloric acid is the reagent that provides chloride ions.

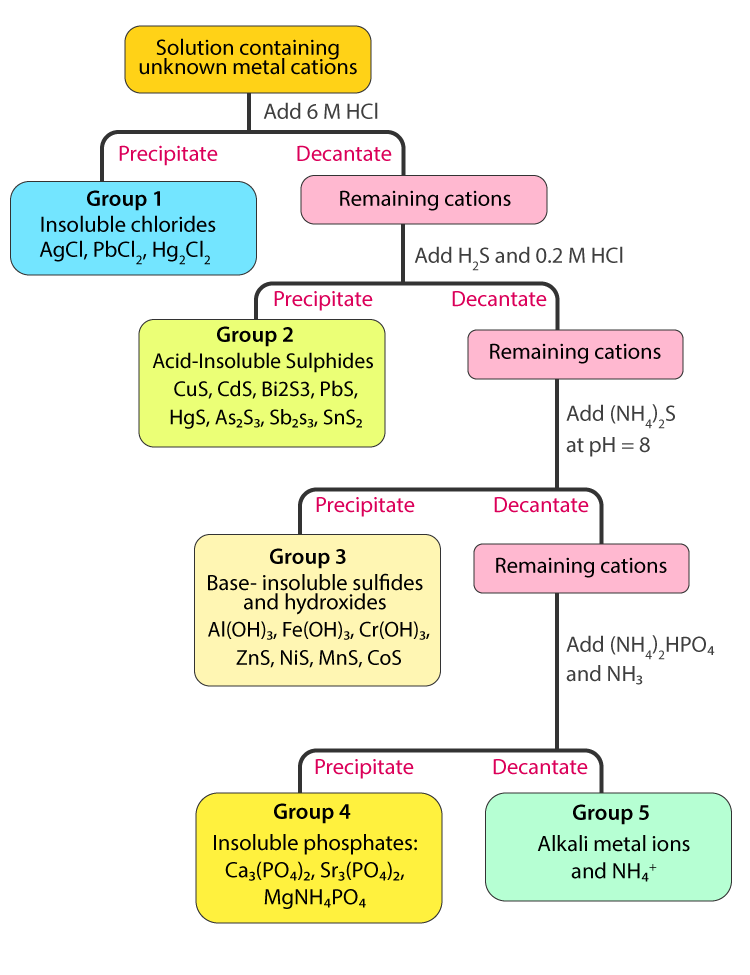
3 1 Separation Of Group I Cations Chemistry Libretexts This page titled 1.5: separation of cations in groups is shared under a public domain license and was authored, remixed, and or curated by muhammad arif malik. cations commonly found in water are separated into five groups by adding suitable reagents that selectively precipitate a set of cations. group i is separated as insoluble chlorides. The introductory course in analytical chemistry is the ideal place in the undergraduate chemistry curriculum for exploring topics such as experimental design, sampling, calibration strategies, standardization, optimization, statistics, and the validation of experimental results. analytical methods come and go, but best practices for designing and validating analytical methods are universal. 15. 3.5: ionic nomenclature is shared under a license and was authored, remixed, and or curated by libretexts. each ionic compound has its own unique name that comes from the names of the ions. after learning a few more details about the names of individual ions, you will be a step away from knowing how to …. Disposal: dispose the ag reaction mixture in the specially labeled for cation group separation "silver, ag" container and not in the sink! group ii separation. transfer the supernatant to another test tube. add 6 m naoh until basic (ph= 8 10) to universal ph paper. mix well. add 4 drops of 6 m naoh in excess.
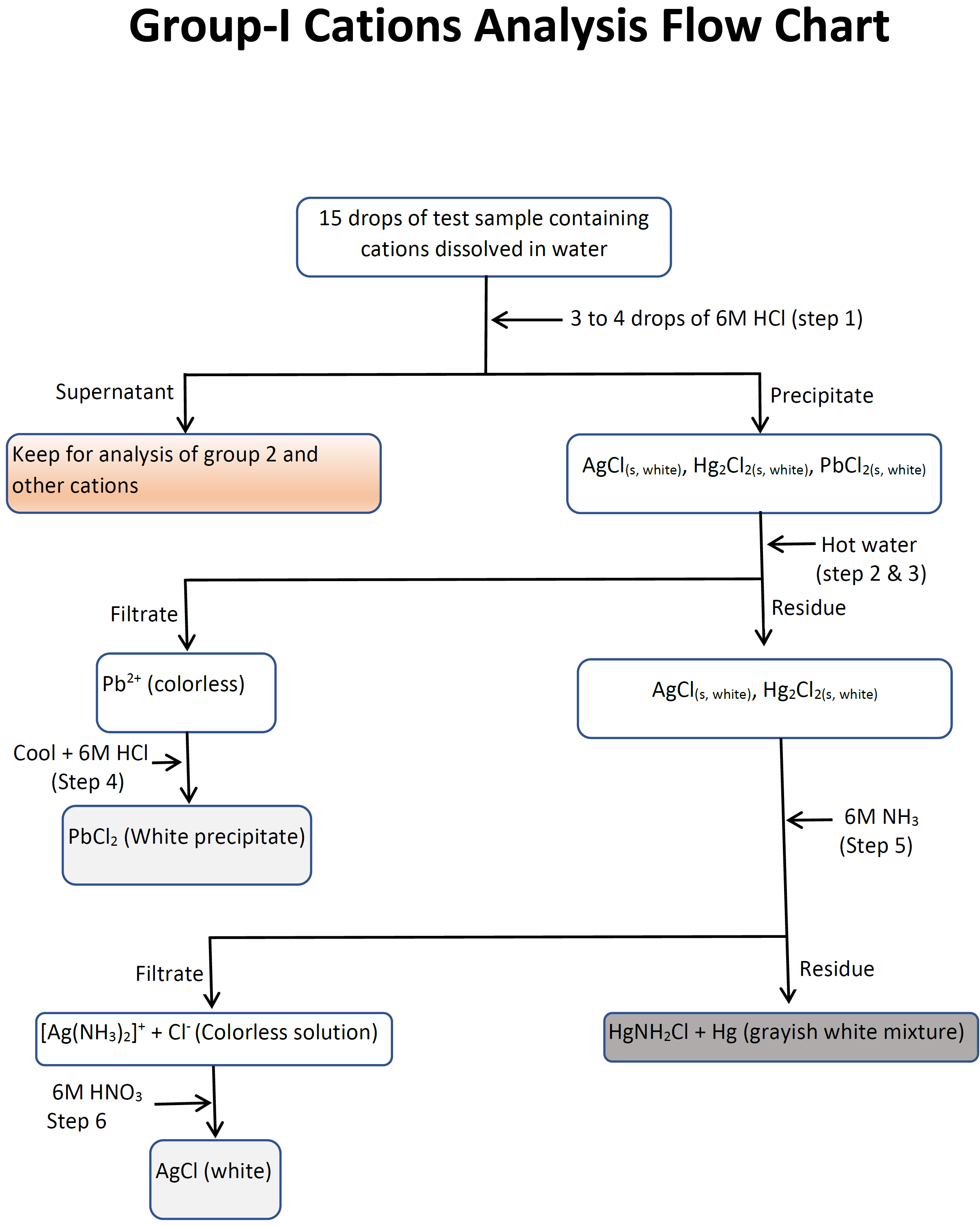
3 3 Procedure Flowchart And Datasheets For Separation And 15. 3.5: ionic nomenclature is shared under a license and was authored, remixed, and or curated by libretexts. each ionic compound has its own unique name that comes from the names of the ions. after learning a few more details about the names of individual ions, you will be a step away from knowing how to …. Disposal: dispose the ag reaction mixture in the specially labeled for cation group separation "silver, ag" container and not in the sink! group ii separation. transfer the supernatant to another test tube. add 6 m naoh until basic (ph= 8 10) to universal ph paper. mix well. add 4 drops of 6 m naoh in excess. Part b: analysis and identification of group i cations in an unknown sample. obtain a test tube which contains a mixture of group i cations. record the id code of the sample on your report form. pour 1.0 ml of the above mixture into a second small test tube and then add 2 drops of 6 m hcl to this test tube. 5. group v cations (mg 2 , na , k and nh 4 ): none of the cations in this group form precipitates in the separation processes of group 1 4 cations and thus remain in the final solution. the flowchart for separating the five groups of cations is given below. classification of cations.

3 1 Separation Of Group I Cations Chemistry Libretexts Part b: analysis and identification of group i cations in an unknown sample. obtain a test tube which contains a mixture of group i cations. record the id code of the sample on your report form. pour 1.0 ml of the above mixture into a second small test tube and then add 2 drops of 6 m hcl to this test tube. 5. group v cations (mg 2 , na , k and nh 4 ): none of the cations in this group form precipitates in the separation processes of group 1 4 cations and thus remain in the final solution. the flowchart for separating the five groups of cations is given below. classification of cations.
Cation And Anion Diagram

Comments are closed.