5 1 Separation Of Group Iii Cations Chemistry Libretexts
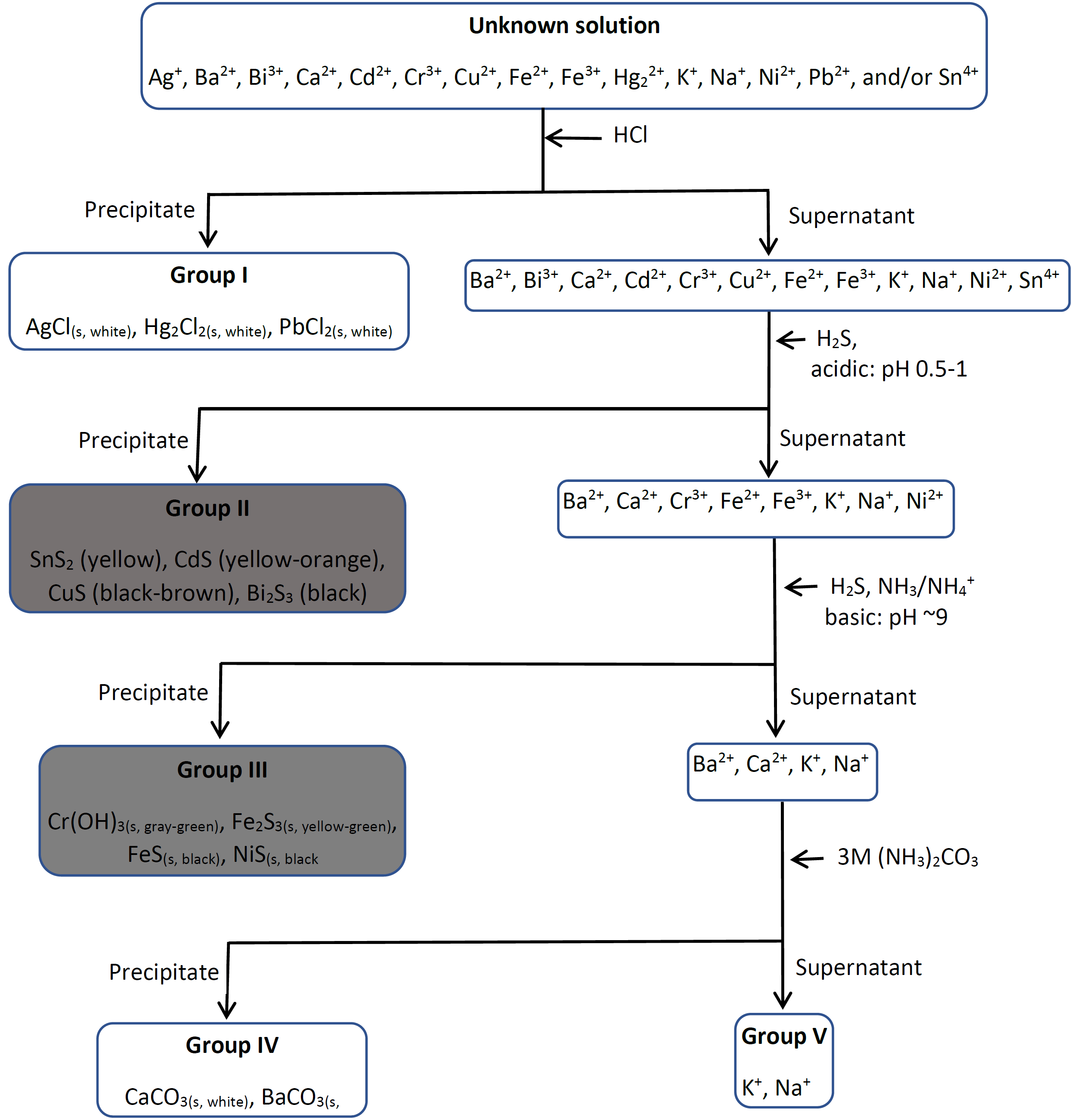
1 5 Separation Of Cations In Groups Chemistry Libretexts Figure 5.1.1: precipitation of iron ions and chromium ions, as fe(oh) 3, fe(oh) 2, and cr(oh) 3 in the presence by oh − at ph ~9. the concentration of fe2 , i.e., the most soluble hydroxide of group iii cations, is reduced by more than 99.99%, i.e., from 0.1m to 4.9 x 10 7 m when ph is increased to 9 and oh − concentration is increased to. 5.1: separation of group iii cations after insoluble chlorides and acid insoluble sulfides of group i and group ii has been removed, the medium is made alkaline at ph ~9 where group iii cations precipitate out as hydroxides, i.e., iron(ii) hydroxide, iron (iii) hydroxide, chromium(iii) hydroxide, and nickel(ii) hydroxides.

Tipos De Cationes This page titled 1.5: separation of cations in groups is shared under a public domain license and was authored, remixed, and or curated by muhammad arif malik. cations commonly found in water are separated into five groups by adding suitable reagents that selectively precipitate a set of cations. group i is separated as insoluble chlorides. 5. group v cations (mg 2 , na , k and nh 4 ): none of the cations in this group form precipitates in the separation processes of group 1 4 cations and thus remain in the final solution. the flowchart for separating the five groups of cations is given below. classification of cations. E same time for the presence of group iii cations and use about 20 24 drops in your analysis.step 2: oxidation of cr(iii) to cr(vi) an. separation of insoluble hydroxides: add 1 ml. of 6 m naoh to the solution in a 30 ml beaker. boil v. ry gently for 1 minute while stirring. remove heat and slo. y add dropwise 1 ml of 1 m nac. Table 3.4.1 3.4. 1 lists the elements that use the common system, along with their respective cation names. the name of a monatomic anion consists of the stem of the element name, the suffix ide, and then the word ion. thus, as we have already seen, cl − is “chlor ” “ ide ion,” or the chloride ion.

5 3 Procedure Flowchart And Datasheets For Separation And E same time for the presence of group iii cations and use about 20 24 drops in your analysis.step 2: oxidation of cr(iii) to cr(vi) an. separation of insoluble hydroxides: add 1 ml. of 6 m naoh to the solution in a 30 ml beaker. boil v. ry gently for 1 minute while stirring. remove heat and slo. y add dropwise 1 ml of 1 m nac. Table 3.4.1 3.4. 1 lists the elements that use the common system, along with their respective cation names. the name of a monatomic anion consists of the stem of the element name, the suffix ide, and then the word ion. thus, as we have already seen, cl − is “chlor ” “ ide ion,” or the chloride ion. Figure 9.4.4: the potential energy of a sodium ion in a nacl crystal for n = 8. the equilibrium bond length occurs when the energy is a minimized. as long as n> 1, the curve for u has the same general shape: u approaches infinity as r → 0 and u approaches zero as r → ∞. Mix well. add 4 drops of 6 m naoh in excess. mix well, heat in the hot water bath to just below boiling for 5 minutes. centrifuge. (make sure you use a counter balance when you centrifuge). if the precipitate is deep blue, cu2 , a cation of group ii, is present as cu(oh)2, or if you obtained black ppt, cuo.
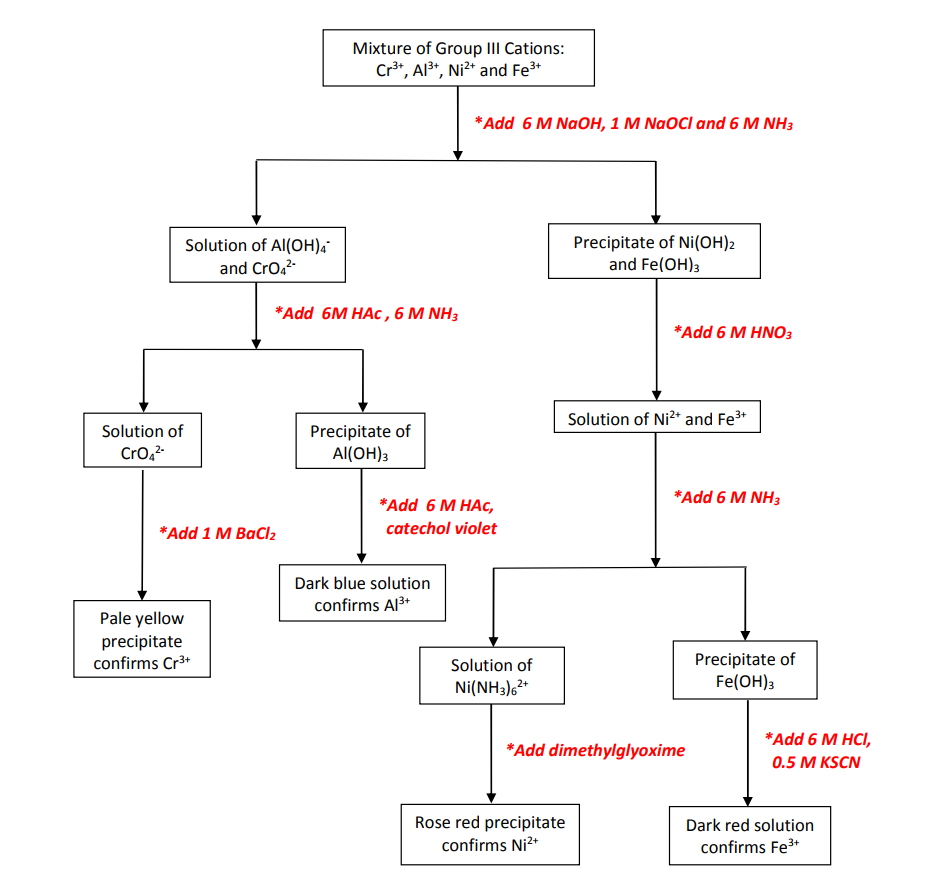
7 Qualitative Analysis Of Group Iii Ions Experiment Chemistry Figure 9.4.4: the potential energy of a sodium ion in a nacl crystal for n = 8. the equilibrium bond length occurs when the energy is a minimized. as long as n> 1, the curve for u has the same general shape: u approaches infinity as r → 0 and u approaches zero as r → ∞. Mix well. add 4 drops of 6 m naoh in excess. mix well, heat in the hot water bath to just below boiling for 5 minutes. centrifuge. (make sure you use a counter balance when you centrifuge). if the precipitate is deep blue, cu2 , a cation of group ii, is present as cu(oh)2, or if you obtained black ppt, cuo.

Comments are closed.