5 3d Phase Diagrams
Phase Diagrams Chemtalk One component phase diagram. figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists. The critical point remains a point on the surface even on a 3d phase diagram. an orthographic projection of the 3d p – v – t graph showing pressure and temperature as the vertical and horizontal axes collapses the 3d plot into the standard 2d pressure–temperature diagram.

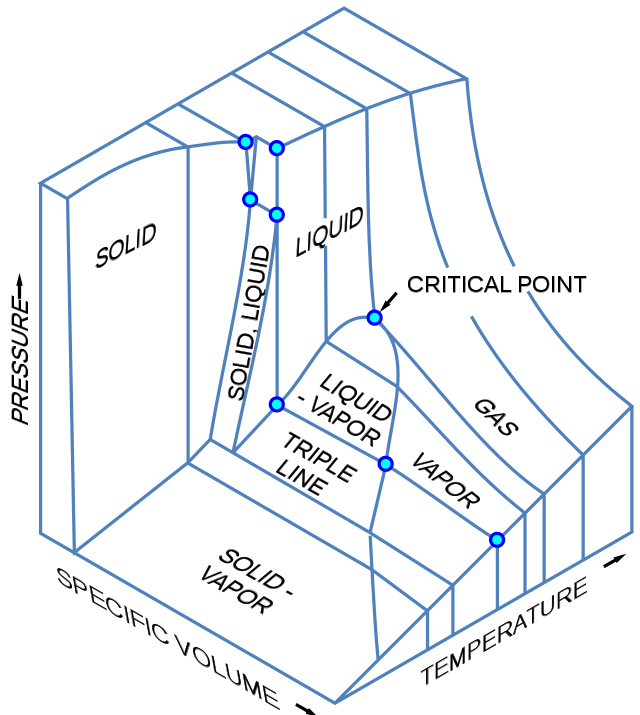
Phase Diagram Definition Explanation And Diagram Three dimensional phase change diagrams plot three thermodynamic variables and show regions of space corresponding to different phases. in this type of diagram, we have a triple line instead of a triple point, and coexistence surfaces instead of coexistence curves. below is a generic 3d diagram plotting temperature, pressure, and specific volume. D = (cp 2) − (c(p − 1) p) d = c − p 2 (chapter 4.7) (chapter 4.8) where d is the degrees of freedom, c is the number of components, p is the number of phases. the 2 comes from t and p as independent variables. so let’s do a couple of examples where we apply the gibbs phase rule! let’s look at the single component phase diagram. Such a p t graph is called a phase diagram. [1] [1] in the phase diagram, we can observe that the boiling point curve (the line that distinguishes liquid and vapour phases) ends at a point, which is called critical point. above the critical point, liquid and gas can no longer be distinguished and are classified as a supercritical fluid. Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram. under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. at 2, it would be a liquid; and at 3, it would be a vapor (a gas).

Phase Diagram Chemistry Dictionary Glossary Such a p t graph is called a phase diagram. [1] [1] in the phase diagram, we can observe that the boiling point curve (the line that distinguishes liquid and vapour phases) ends at a point, which is called critical point. above the critical point, liquid and gas can no longer be distinguished and are classified as a supercritical fluid. Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram. under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. at 2, it would be a liquid; and at 3, it would be a vapor (a gas). Example 12.4.1 12.4. 1: water. referring to the phase diagram of water in figure 12.4.2 12.4. 2: predict the physical form of a sample of water at 400°c and 150 atm. describe the changes that occur as the sample in part (a) is slowly allowed to cool to −50°c at a constant pressure of 150 atm. A 3d phase diagram is a type of graph in which three different conditions (such as p, v, t) are plotted along the cartesian axes. > it shows the conditions at which different phases occur and coexist at equilibrium. the equilibrium conditions are shown as curved surfaces in 3d, with areas for solid, liquid, and vapour phases and areas where two or three phases can coexist in equilibrium. for.
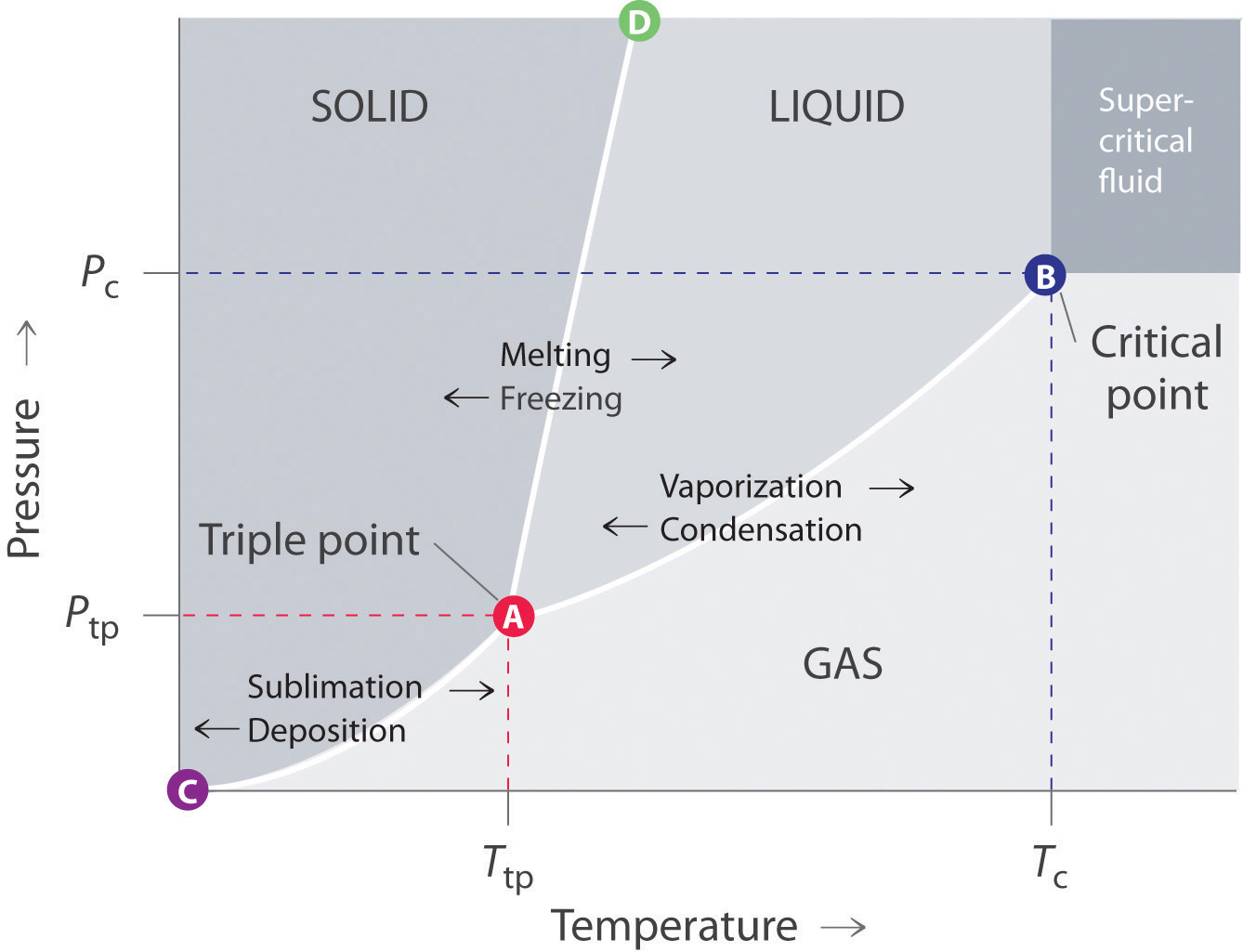
Phase Diagrams Example 12.4.1 12.4. 1: water. referring to the phase diagram of water in figure 12.4.2 12.4. 2: predict the physical form of a sample of water at 400°c and 150 atm. describe the changes that occur as the sample in part (a) is slowly allowed to cool to −50°c at a constant pressure of 150 atm. A 3d phase diagram is a type of graph in which three different conditions (such as p, v, t) are plotted along the cartesian axes. > it shows the conditions at which different phases occur and coexist at equilibrium. the equilibrium conditions are shown as curved surfaces in 3d, with areas for solid, liquid, and vapour phases and areas where two or three phases can coexist in equilibrium. for.

Comments are closed.