5 How To Calculate The Osmolarity Trending Hutomo
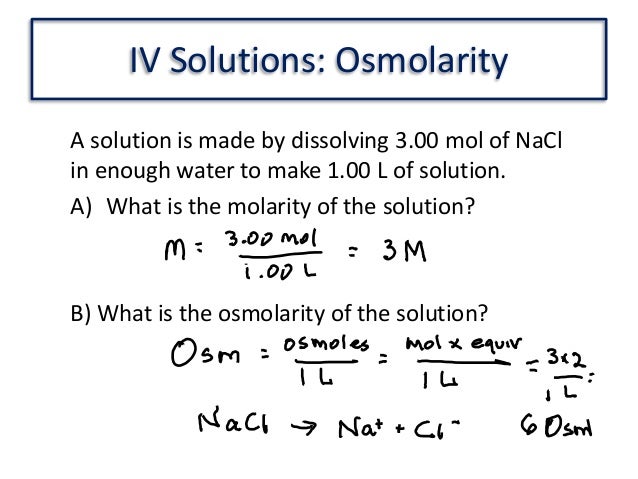
5 How To Calculate The Osmolarity Trending Hutomo To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. convert that number to osmoles per liter and add the osmolarity of each particle together to get the solution's osmolarity. keep reading to learn how to solve any osmolarity problem in just a few simple steps. Solution. set up a table for easy calculation. osmolarity = 141.96 mosmol 562.5ml × 1000ml 1 l = 252 mosmol l. answer link. you multiply the molarity by the number of osmoles that each solute produces. an osmole (osmol) is 1 mol of particles that contribute to the osmotic pressure of a solution. for example, "nacl" dissociates completely in.

5 How To Calculate The Osmolarity Trending Hutomo Osmolarity calculations review | 5 key examples solved. in this video, we'll be looking at five examples of osmolarity calculations solved step by step. the. How to calculate osmolarity. osmolarity, a critical concept in chemistry and biology, plays a pivotal role in various scientific fields. this article will delve into the intricacies of calculating osmolarity, providing a comprehensive guide for both beginners and seasoned professionals. Bun osmolarity = 0.020g 100ml × 1000ml 1 l × 1mol 28.01g × 1 osmol 1mol = 0.0071 osmol l. blood osmolarity = (0.280 0.008 32 0.0071) osmol l = 0.295 osmol l = 295 mosmol l. answer link. you multiply the molarity by the number of osmoles that each solute produces. > an osmole (osmol) is 1 mol of particles that contribute to the osmotic. So, the osmolarity of solution of 5% glucose or dextrose (n' = 1, mw = 180) is equal to 278 mosm l. osmolarity of solution of normal saline (0.9%) with n' = 2 and mw = 58.5 is equal to 308 mosm l. normal saline is considered as an isosmotic solution (i.e. in the range of 275–295). we resolve this issue later by introducing the activity.
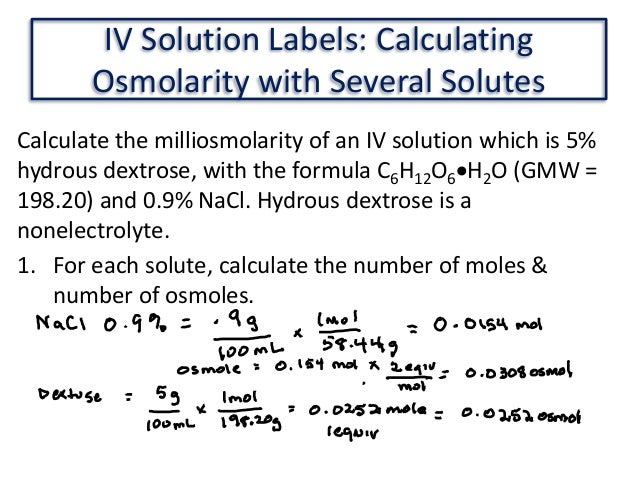
5 How To Calculate The Osmolarity Trending Hutomo Bun osmolarity = 0.020g 100ml × 1000ml 1 l × 1mol 28.01g × 1 osmol 1mol = 0.0071 osmol l. blood osmolarity = (0.280 0.008 32 0.0071) osmol l = 0.295 osmol l = 295 mosmol l. answer link. you multiply the molarity by the number of osmoles that each solute produces. > an osmole (osmol) is 1 mol of particles that contribute to the osmotic. So, the osmolarity of solution of 5% glucose or dextrose (n' = 1, mw = 180) is equal to 278 mosm l. osmolarity of solution of normal saline (0.9%) with n' = 2 and mw = 58.5 is equal to 308 mosm l. normal saline is considered as an isosmotic solution (i.e. in the range of 275–295). we resolve this issue later by introducing the activity. Osmolarity equation. a simple equation for calculating the osmolarity of a solution is derived from the molarity of the solution such that: osmolarity = molarity × n. n is the number of particles or ions present in the solution. for instance a solution of nacl will dissociate into two ions that are na and cl . Table of content [ open ]0.1 osm (osmolarity) = m ( molarity) x number of particles of dissociation.0.2 the sugar in red bull is a mixture of glucose and sucrose (a disaccharide made up of one molecule of glucose and one molecule of fructose bonded together).0.3 after selection, the base osmolarity is displayed.0.4 the serum […].

How To Solve Osmolarity Calculation Problems Youtube Osmolarity equation. a simple equation for calculating the osmolarity of a solution is derived from the molarity of the solution such that: osmolarity = molarity × n. n is the number of particles or ions present in the solution. for instance a solution of nacl will dissociate into two ions that are na and cl . Table of content [ open ]0.1 osm (osmolarity) = m ( molarity) x number of particles of dissociation.0.2 the sugar in red bull is a mixture of glucose and sucrose (a disaccharide made up of one molecule of glucose and one molecule of fructose bonded together).0.3 after selection, the base osmolarity is displayed.0.4 the serum […].

What Is Osmolarity In Biology

Comments are closed.