Acid Base Ph Titration Curves And Equivalence Points Concept
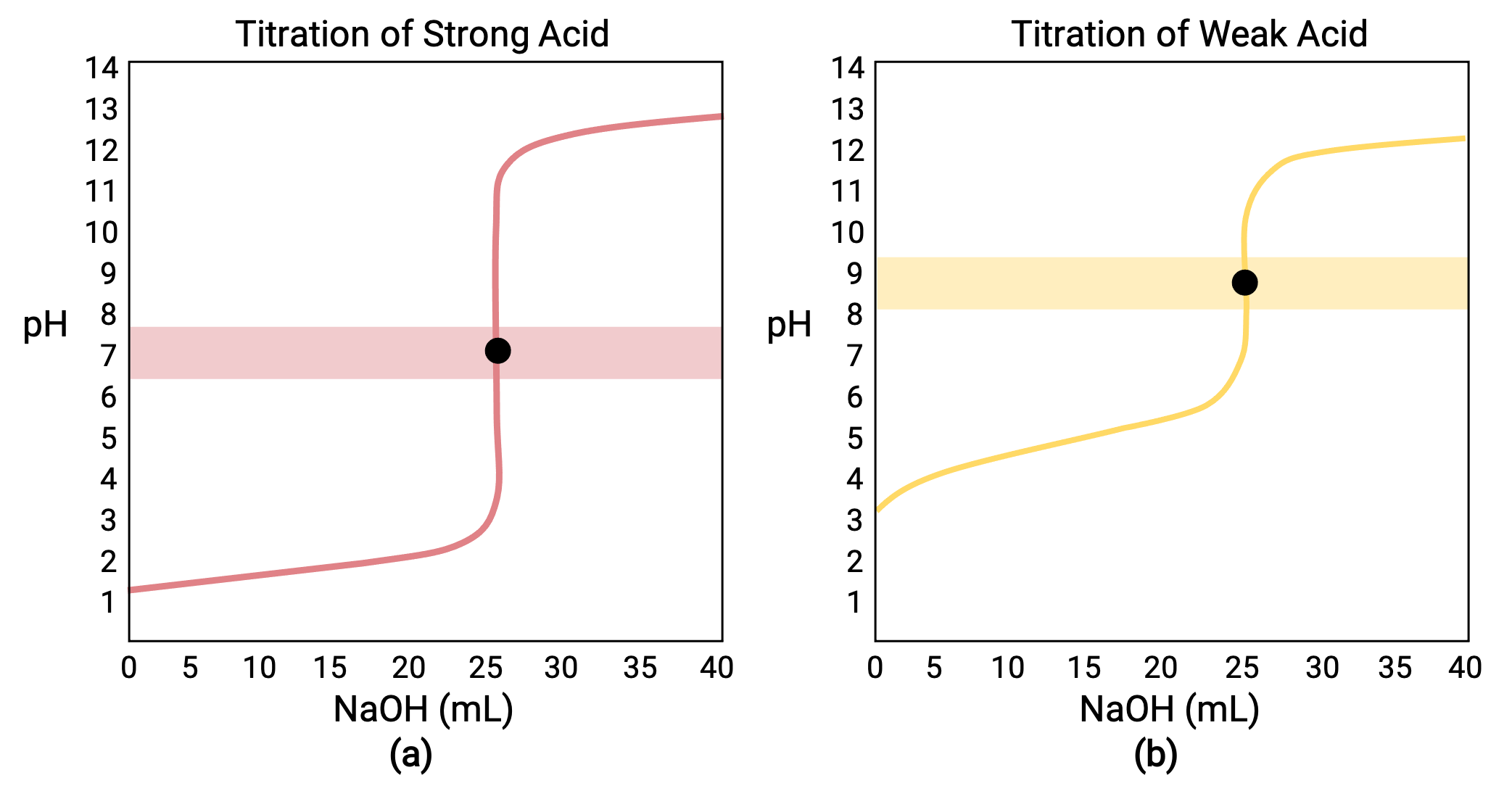
Acid Base Ph Titration Curves And Equivalence Points Concept The pkb of ammonia is 4.75 at 25°c. answer. as shown in part (b) in figure 17.4.3, the titration curve for nh3, a weak base, is the reverse of the titration curve for acetic acid. in particular, the ph at the equivalence point in the titration of a weak base is less than 7.00 because the titration produces an acid. (b)the titration curve for the titration of 25.00 ml of 0.100 m acetic acid (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. the titration of a strong or weak base with a strong acid has a similar s shaped curve; however, the curve is inverted as the ph will start in the basic region and decrease with the.
Titration Of A Weak Base With A Strong Acid Chemwiki The equilibrium between the weak acid and its conjugate base influences the ph at the equivalence point, resulting in a slightly basic endpoint. understanding the dissociation constants and equilibrium reactions becomes crucial for accurately determining the unknown concentration. 3. strong acid weak base. in this titration, a strong acid, like. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. the titration of a weak acid with a strong base (or of a weak base with a strong acid) is somewhat more complicated than that just discussed, but it follows the same general principles. Titration curve – a plot of ph vs millilitres of titrant showing the manner in which ph changes vs millilitres of titrant during an acid base titration. equivalence point – the point at which just an adequate reagent is added to react completely with a substance. buffer solution – a solution that resists changes in ph even when a strong. The extent of the jump in the ph at the equivalence point is determined by a combination of factors. in the case of a weak acid, for example, the initial ph is likely to be higher, so the titration curve starts higher. further, the weaker the acid, the stronger will be its conjugate base, so the higher will be the ph at the equivalence point.

Comments are closed.