Acid Base Titration Curves
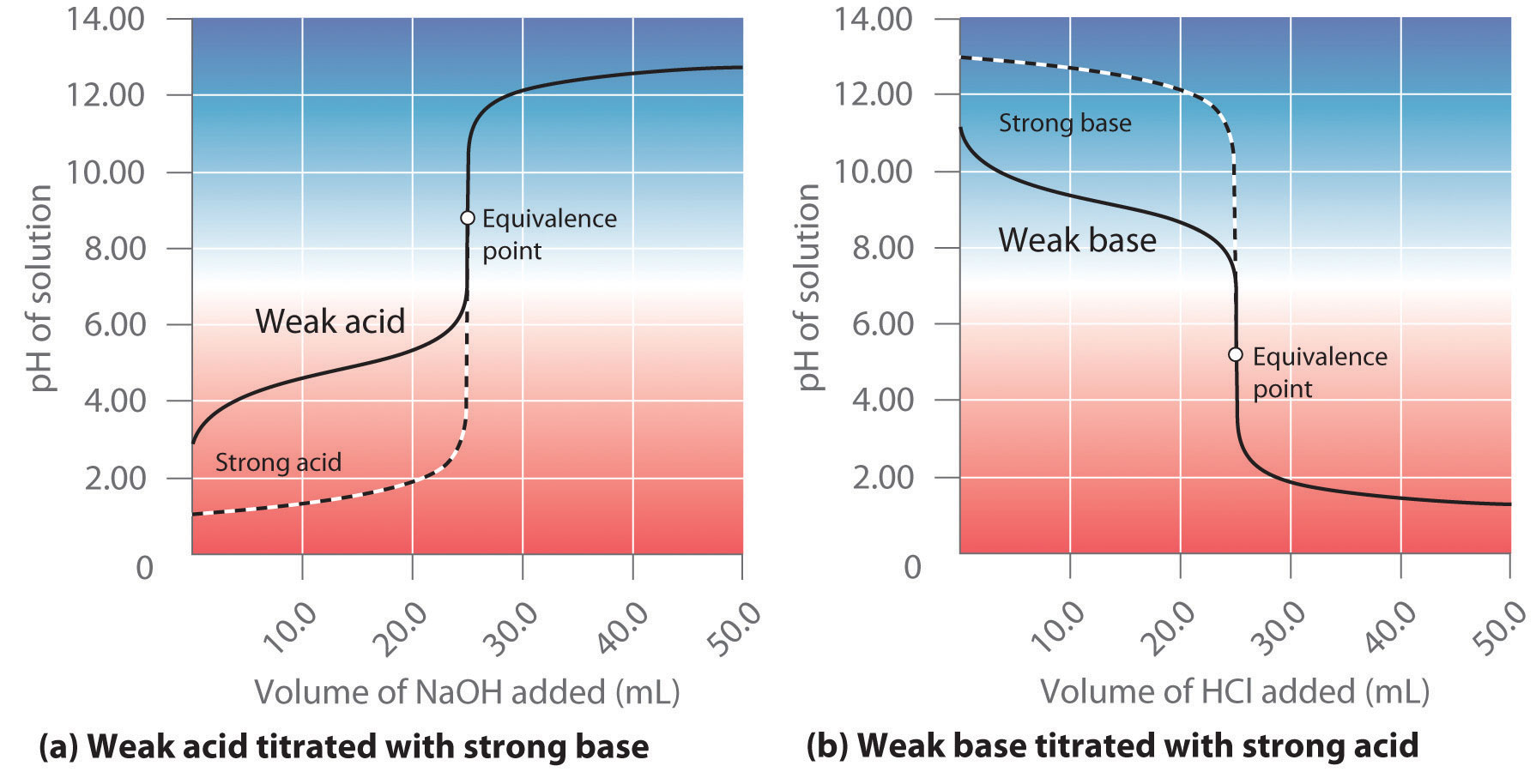
15 6 Acid Base Titration Curves Chemistry Libretexts Plots of acid–base titrations generate titration curves that can be used to calculate the ph, the poh, the \(pk a\), and the \(pk b\) of the system. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. Calculating ph for titration solutions: strong acid strong base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). calculate the ph at these volumes of added base solution: (a) 0.00 ml. (b) 12.50 ml. (c) 25.00 ml.
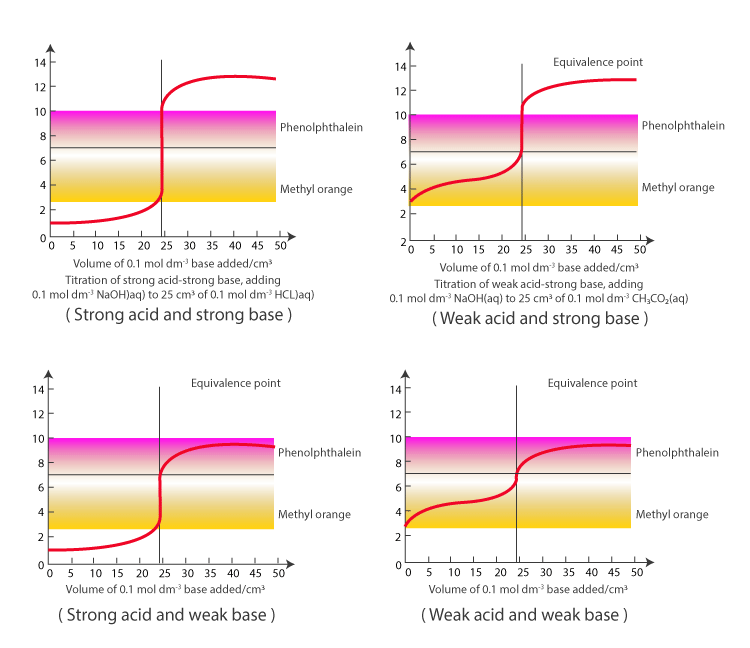
Strong Acid And Base Titration Curve Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. see the different types of acid base titrations and their curves, and how to use indicators to identify the equivalence point. This also corresponds to the color change of the indicator. figure 21.19.1 21.19. 1: a titration curve shows the ph changes that occur during the titration of an acid with a base. on the left, base is being added to acid. on the right, acid is being added to base. in both cases, the equivalence point is at ph 7. A ph electrode is the obvious sensor for monitoring an acid–base titration and the result is a potentiometric titration curve. for example, figure 9.2.9 a shows a small portion of the potentiometric titration curve for the titration of 50.0 ml of 0.050 m ch 3 cooh with 0.10 m naoh, which focuses on the region that contains the equivalence point. An acid–base titration is a method of quantitative analysis for determining the concentration of brønsted lowry acid or base (titrate) by neutralizing it using a solution of known concentration (titrant). [1] a ph indicator is used to monitor the progress of the acid–base reaction and a titration curve can be constructed.
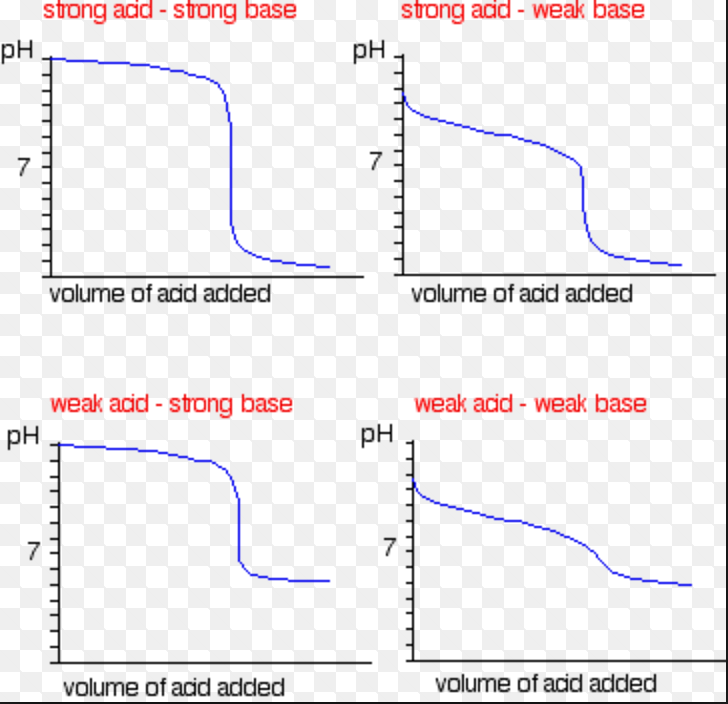
Acid Base Titration Using Indicator Chemistry Class 11 Ionic A ph electrode is the obvious sensor for monitoring an acid–base titration and the result is a potentiometric titration curve. for example, figure 9.2.9 a shows a small portion of the potentiometric titration curve for the titration of 50.0 ml of 0.050 m ch 3 cooh with 0.10 m naoh, which focuses on the region that contains the equivalence point. An acid–base titration is a method of quantitative analysis for determining the concentration of brønsted lowry acid or base (titrate) by neutralizing it using a solution of known concentration (titrant). [1] a ph indicator is used to monitor the progress of the acid–base reaction and a titration curve can be constructed. Learn how to determine the concentration of an acid or base by measuring the volume of titrant that reacts with it. find out the types of acid base titration, the titration curve, the equivalence point and the choice of indicators for each type. Example 15.7.1: calculating ph for titration solutions: strong acid strong base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh the titration curve is shown in figure 15.7.1 (below). calculate the ph at these volumes of added base solution: 0.00 ml. 12.50 ml.

Comments are closed.