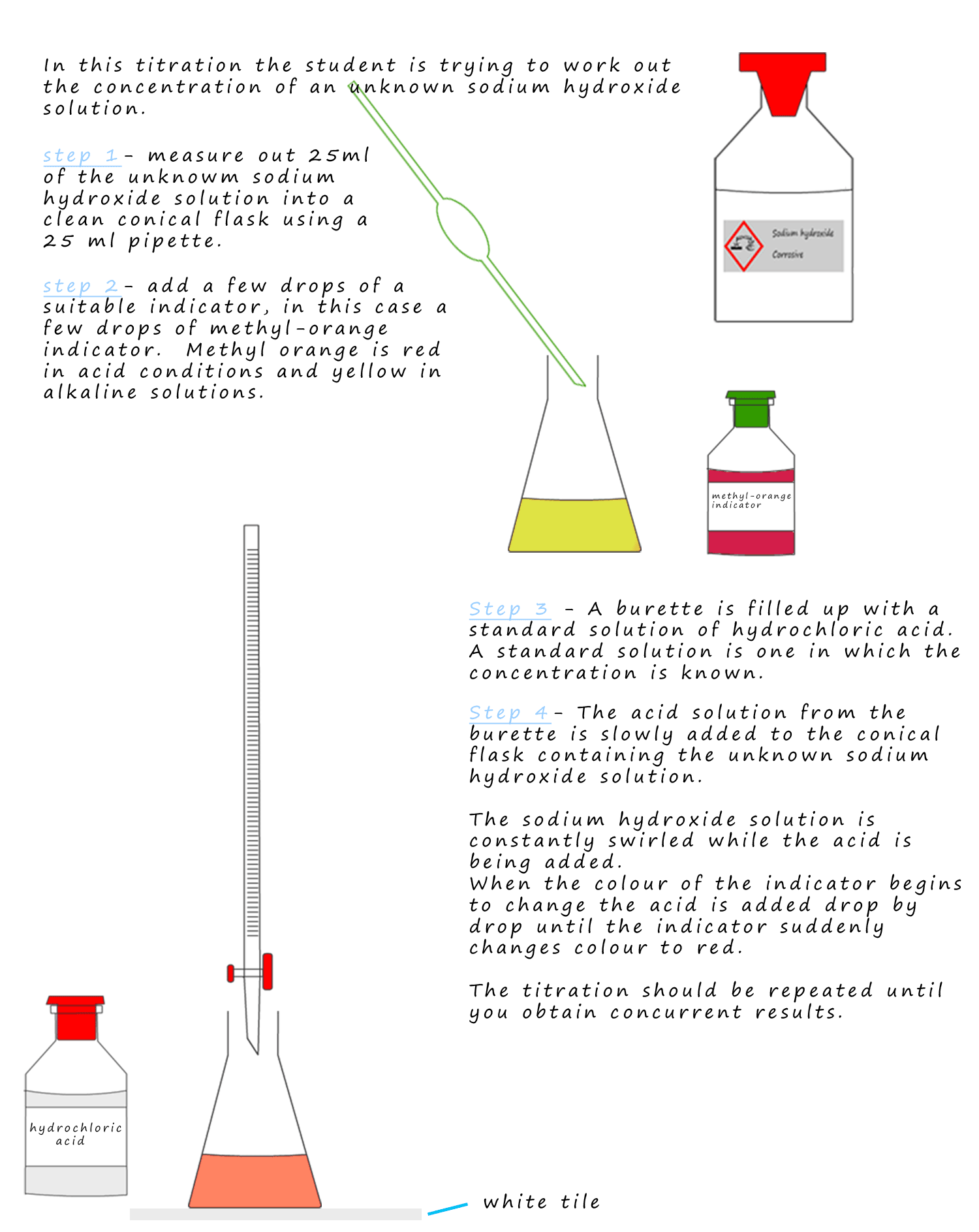
Acid Base Titrations Mcguire Chemistry Libretexts Vro Vrogue Co
Acid Base Titrations Mcguire Chemistry Libretexts Vro Vrogue Co Some titrations requires the solution to be boiled due to the \(co 2\) created from the acid base reaction. the \(co 2\) forms carbonic acid (\(h 2co 3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. after boiling water, most of the \(co 2\) will be removed from the solution allowing the solution to be. Given that k al = 1.5 x 10 3 and k a2 = 2.0 x 10 6 for malonic acid, find the ph at each of the labelled points. 1) point a: 2) point b: 3) point c: 4) point d: 5) point e: 25.0 ml of a 0.10 m solution of a weak base (pk b1 = 4.50 and pk b2 = 7.24) is titrated with 0.10 m hcl and the following titration curve is observed.

Acid Base Titrations Mcguire Chemistry Libretexts Vro Vrogue Co Before 1800, most acid–base titrations used h 2 so 4, hcl, or hno 3 as acidic titrants, and k 2 co 3 or na 2 co 3 as basic titrants. a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing \(\text{co} 3^{2 }\). Calculating ph for titration solutions: strong acid strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). calculate the ph at these volumes of added base solution: (a) 0.00 ml (b) 12.50 ml (c) 25.00 ml (d) 37.50 ml. solution. Acid–base titrimetry is a standard method for the quantitative analysis of many inorganic acids and bases. a standard solution of naoh can be used to determine the concentration of inorganic acids, such as h 3 po 4 or h 3 aso 4, and inorganic bases, such as na 2 co 3 can be analyzed using a standard solution of hcl. Weak base strong acid titration curves a weak base strong acid titration curve. this type of titration would yield a curve with decreasing ph, given that you’re neutralizing base with a strong acid: (7) where ha is the conjugate acid of the original weak base a – and is therefore a weak base. a few features can be found … the flip side of.

Comments are closed.