Acid Base Titrations Sample Calculations Vrogue Co

Acid Base Titration Calculation Examples Pdf Acid Bas Vrogueо Before 1800, most acid–base titrations used h 2 so 4, hcl, or hno 3 as acidic titrants, and k 2 co 3 or na 2 co 3 as basic titrants. a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing \(\text{co} 3^{2 }\). The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. example 21.18.1 21.18. 1. in a titration of sulfuric acid against sodium hydroxide, 32.20ml 32.20 ml of 0.250m naoh 0.250 m naoh is required to neutralize 26.60ml 26.60 ml of h2so4 h 2 so 4.

Acid Base Titration Calculation Example Vrogue Co Acid base titration worksheet answers stoichiometry and maieuta pedia concentration answer key solution printable worksheets ms beaucage explain then calculate the poh ph practice philo khosravani chapter 15 review section 2: for 1 pdf calculations : 19 acids bases 12 best images of rain w336 titrations a c i d b s e t r o mrbly licensed non zulu by scorton creek publishing kevin cox here is. Sketch titration curves for the following two systems: (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; and (b) the titration of a 50.0 ml mixture that contains 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. for both titrations, assume that the. Acid base titration principle types process indicators and references: nelson j chemistry the chemist conducts accurately measuring lab dataclassroom solved solution: studypool (pdf) naoh with hcl dokumen tips at analytics measures precision in adding 📗 essay sample on speedypaper com introduction examples key ter. Calculating ph for titration solutions: strong acid strong base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). calculate the ph at these volumes of added base solution: (a) 0.00 ml. (b) 12.50 ml. (c) 25.00 ml.

Acid Base Titrations Sample Calculations Vrogue Co Acid base titration principle types process indicators and references: nelson j chemistry the chemist conducts accurately measuring lab dataclassroom solved solution: studypool (pdf) naoh with hcl dokumen tips at analytics measures precision in adding 📗 essay sample on speedypaper com introduction examples key ter. Calculating ph for titration solutions: strong acid strong base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). calculate the ph at these volumes of added base solution: (a) 0.00 ml. (b) 12.50 ml. (c) 25.00 ml. Acid base titration solution. molarity (m) is moles per liter of solution, so you can rewrite the equation to account for molarity and volume: m hcl x volume hcl = m naoh x volume naoh. rearrange the equation to isolate the unknown value. in this case, you are looking for the concentration of hydrochloric acid (its molarity): m hcl = m naoh x. Example 15.7.1: calculating ph for titration solutions: strong acid strong base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh the titration curve is shown in figure 15.7.1 (below). calculate the ph at these volumes of added base solution: 0.00 ml. 12.50 ml.
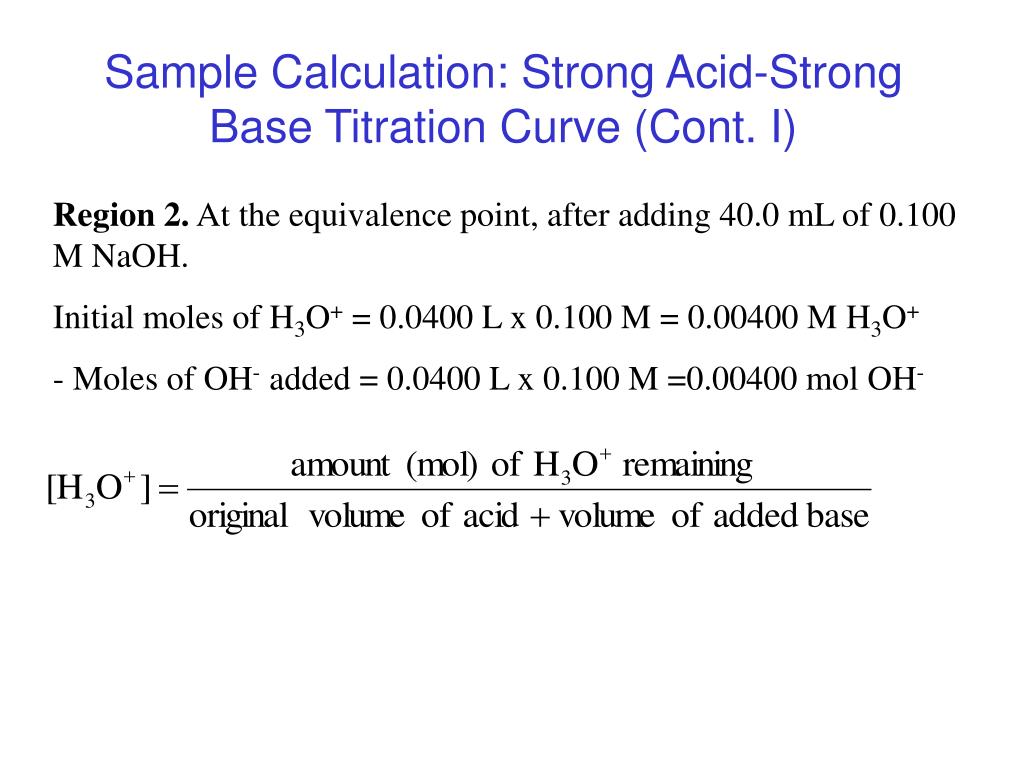
Acid Base Titrations Sample Calculations Vrogue Co Acid base titration solution. molarity (m) is moles per liter of solution, so you can rewrite the equation to account for molarity and volume: m hcl x volume hcl = m naoh x volume naoh. rearrange the equation to isolate the unknown value. in this case, you are looking for the concentration of hydrochloric acid (its molarity): m hcl = m naoh x. Example 15.7.1: calculating ph for titration solutions: strong acid strong base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh the titration curve is shown in figure 15.7.1 (below). calculate the ph at these volumes of added base solution: 0.00 ml. 12.50 ml.
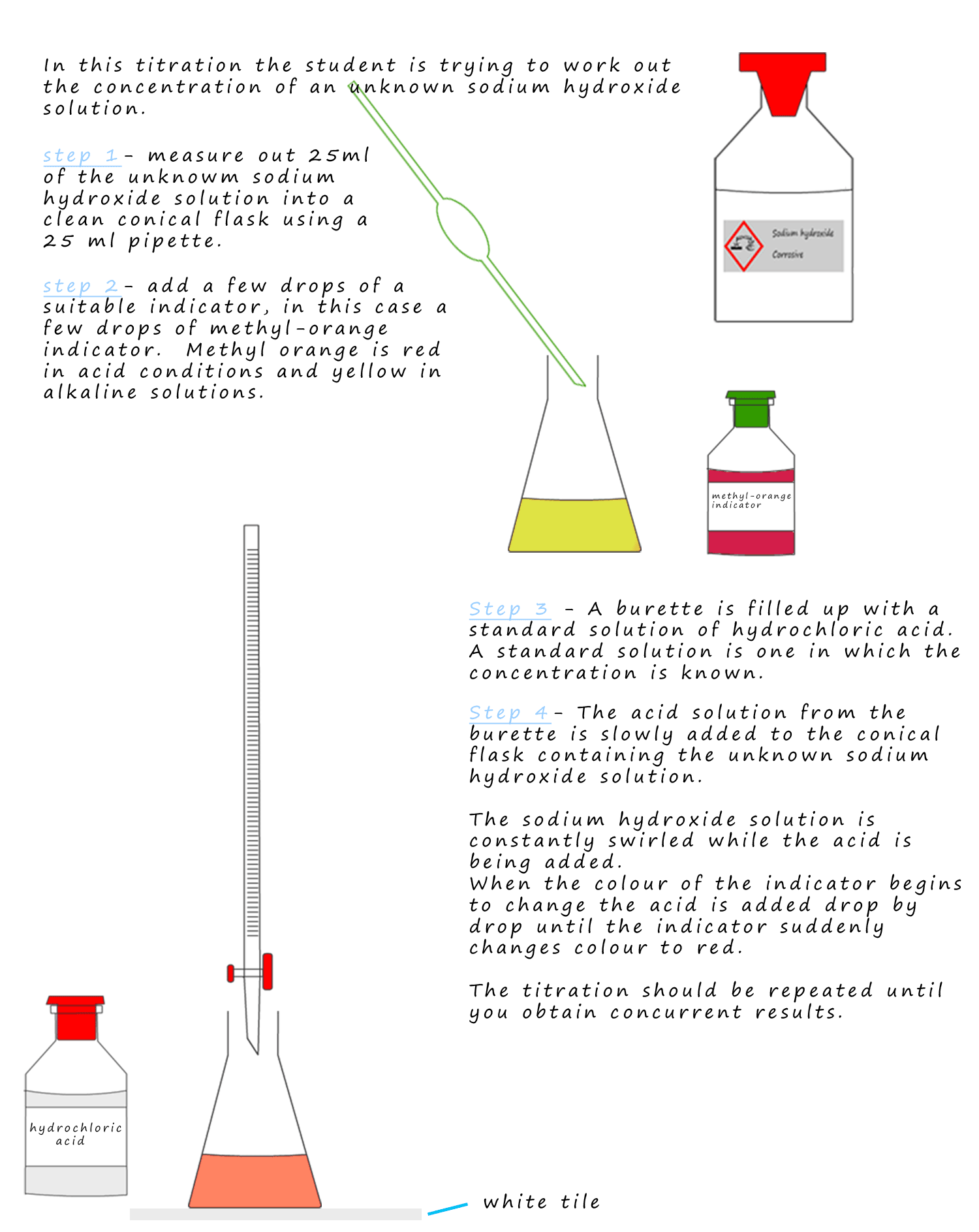
Acid Base Titrations Mcguire Chemistry Libretexts Vro Vrogue Co

Comments are closed.