Acids Bases And Salts Cie Igcse 0620 Ppq
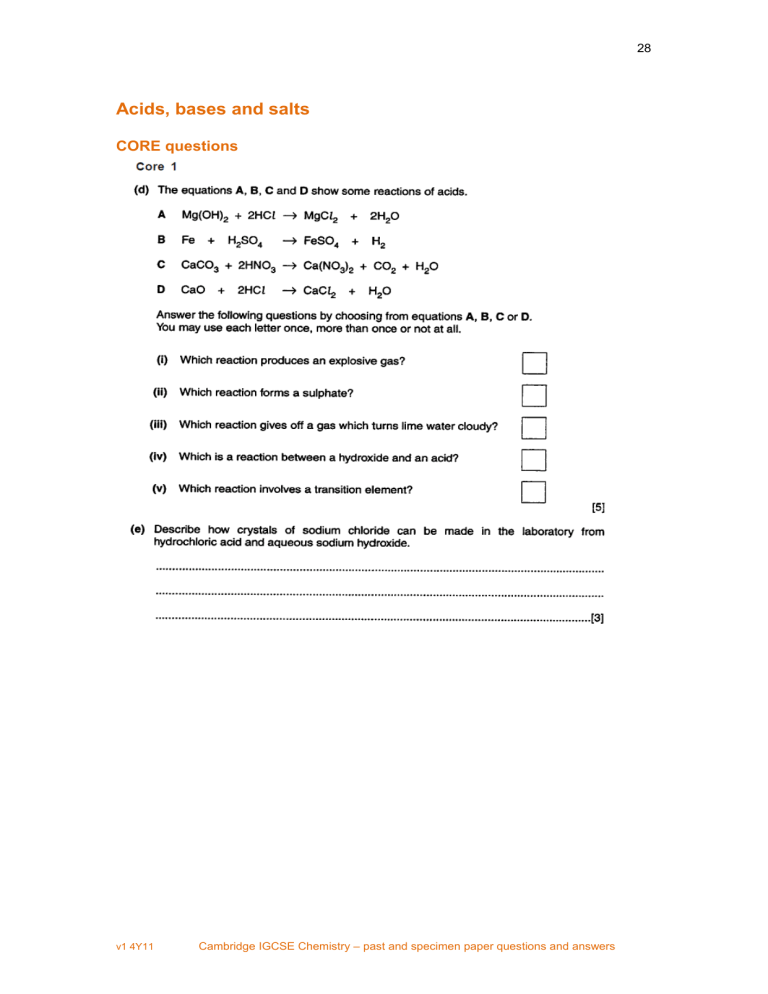
Acids Bases And Salts Cie Igcse 0620 Ppq Acids, bases and salts core questions. alternative to practical question 0620 cambridge igcse chemistry specimen papers (2016) paper 1 question 11. alternative to practical question. 0620 cambridge igcse chemistry specimen papers (2016) paper 2 question 22 paper 2 question 23. paper 1 question; paper 1 question; paper 1 question; paper 1. Acids, bases and salts cie igcse 0620 ppq. 28 acids, bases and salts core questions v1 4y11 cambridge igcse chemistry – past and specimen paper questions and answers 29 v1 4y11 cambridge igcse chemistry – past and specimen paper questions and answers 30 v1 4y11 cambridge igcse chemistry – past and specimen paper questions and answers 31.

Solution Cie Igcse Chemistry 0620 Acids Bases Salts Past Paper Caie igcse chemistry revision. advertisement papers 1 4. topic 1: states of matter. acids, bases and salts. topic 8: the periodic table. topic 9: metals. topic 10:. The burette was emptied and rinsed with distilled water, and then with acid b. this acid was discarded. the burette was then fi lled up to the 0.0 cm3 mark with acid b. (b) experiment 2 experiment 1 was repeated using acid b instead of acid a. use the thermometer diagrams in the table to record the temperatures. 0.0 30 25 20 40 35 30 45 40 35. Acids, bases and salts . question paper 7 . level igcse subject chemistry exam board cie topic acids, bases and salts sub topic paper type alternative to practical booklet question paper 7 . time allowed: 53 minutes. score: 44 . percentage: 100 è. 1. they react with metals above lead in the reactivity series to give hydrogen. acid metal > salt hydrogen. 2. they react with carbonates to give carbon dioxide. acid carbonate > salt water carbon dioxide. 3. they react with bases and alkalis to form a salt and water only. acid base alkali > salt water.
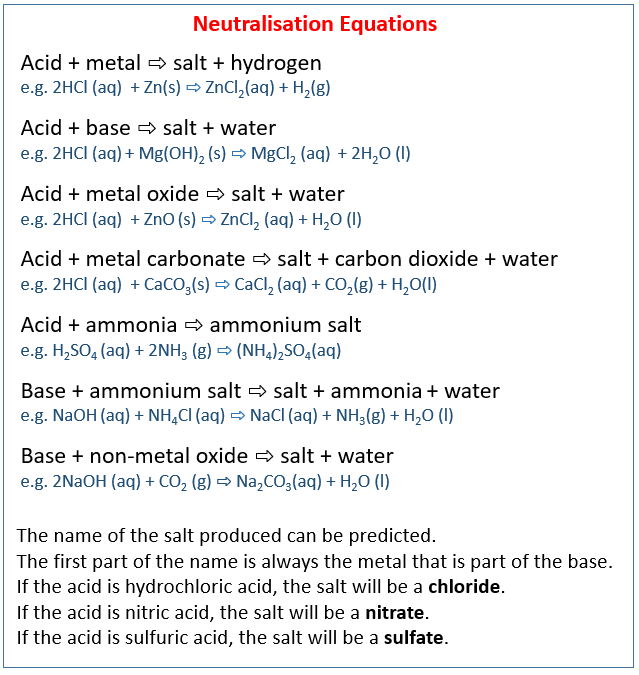
Acid Bases Salts Igcse Chemistry Solutions Examples Worksheets Acids, bases and salts . question paper 7 . level igcse subject chemistry exam board cie topic acids, bases and salts sub topic paper type alternative to practical booklet question paper 7 . time allowed: 53 minutes. score: 44 . percentage: 100 è. 1. they react with metals above lead in the reactivity series to give hydrogen. acid metal > salt hydrogen. 2. they react with carbonates to give carbon dioxide. acid carbonate > salt water carbon dioxide. 3. they react with bases and alkalis to form a salt and water only. acid base alkali > salt water. Another name for a negative ion. balanced equation. a chemical equation in which the number of each type of atom is the same on both sides of the arrow. base. a metal oxide or hydroxide; a base will neutralise an acid, to form a salt and water. study with quizlet and memorize flashcards containing terms like acidic solution, acid, alkali metals. A adding calcium hydroxide to acid soil. b adding citric acid to sodium hydrogen carbonate solution. c adding sodium chloride to silver nitrate solution. d adding sodium hydroxide to hydrochloric acid. 6. the oxide of element x was added to an acid. it reacted to form a salt and water. oxide of element x. acid.

Free Printable Acids Bases And Salts Worksheets Another name for a negative ion. balanced equation. a chemical equation in which the number of each type of atom is the same on both sides of the arrow. base. a metal oxide or hydroxide; a base will neutralise an acid, to form a salt and water. study with quizlet and memorize flashcards containing terms like acidic solution, acid, alkali metals. A adding calcium hydroxide to acid soil. b adding citric acid to sodium hydrogen carbonate solution. c adding sodium chloride to silver nitrate solution. d adding sodium hydroxide to hydrochloric acid. 6. the oxide of element x was added to an acid. it reacted to form a salt and water. oxide of element x. acid.
-768.png)
Acids Bases And Salts Definition Types Properties And Uses

Comments are closed.