Acids Bases And Salts Class 7 Science Notes Chapter 5 Reliable
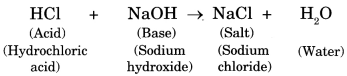
Acids Bases And Salts Class 7 Science Notes Chapter 5 Reliable Salts. salt is the product formed from the neutralisation reaction of acids and bases. in the reaction between hydrochloric acid and sodium hydroxide, the salt formed is sodium chloride. hcl n aoh→n acl h2o. salt can be acidic, basic or neutral in nature. to know more about salts, visit here. Chapter 5 acids, bases and salts notes explained in details get comprehensive notes on class 7 science chapter 5: acids, bases, and salts. understand their properties, everyday uses, and reactions. learn about neutralization, natural indicators, and safety precautions. simple explanations and examples make chemistry easy and fun for students. perfect for revising and mastering the chapter.
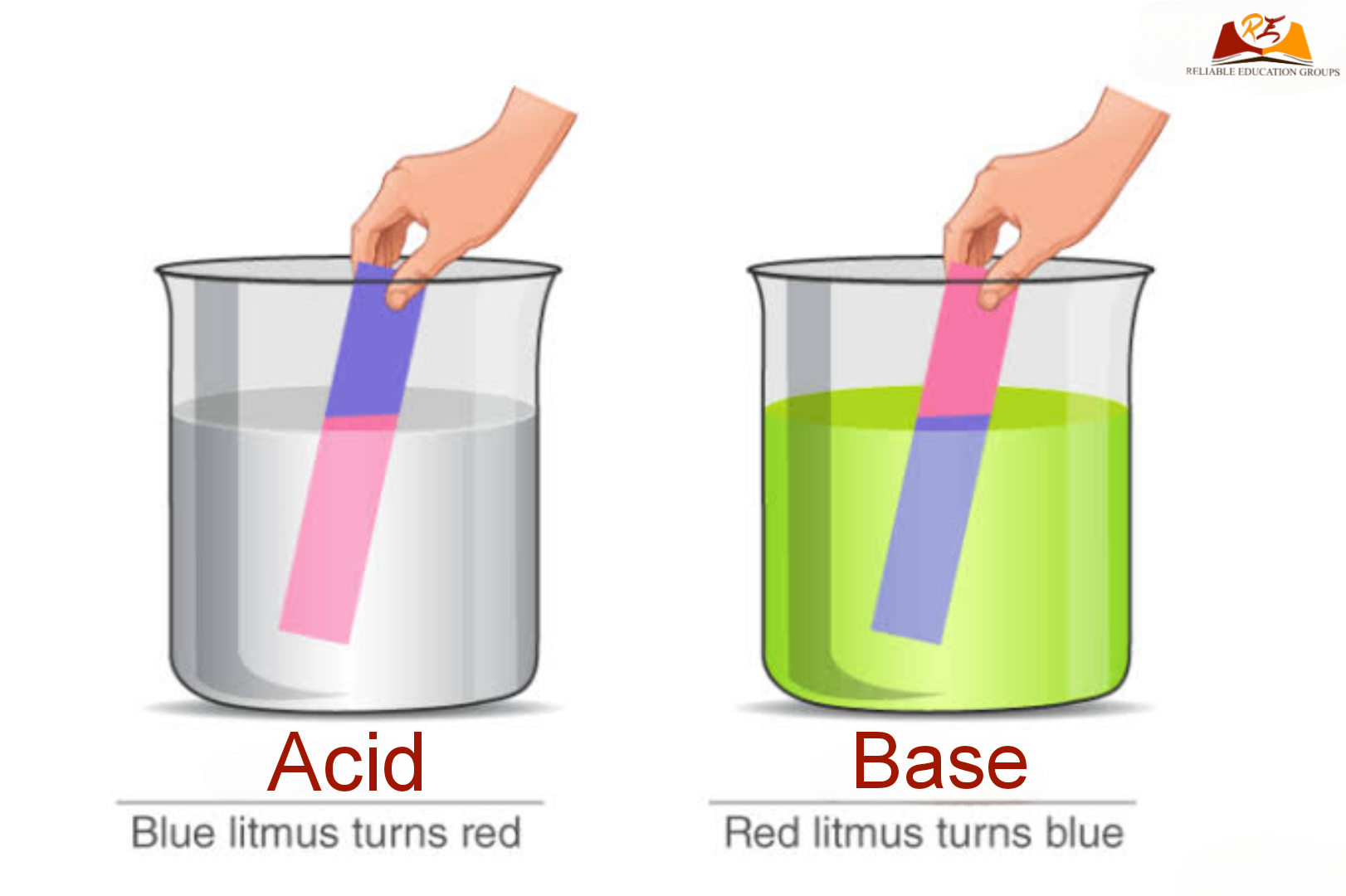
Acids Bases And Salts Class 7 Notes Science Chapter Cbse class 7 science notes chapter 5 acids, bases and salts. in our daily life, we use a large number of edible substances such as lemon, baking soda, tamarind, common salt, sugar, curd and vinegar. some of these substances taste sour, some taste bitter, some taste sweet and some taste salty. acids, bases and salts are the three important. This reaction is known as neutralization reaction. → water and salt are produced as products during the neutralization reaction. → heat is also produced during the neutralization reaction. acid base → salt water heat. → the salt produced during neutralization reactions can be acidic, basic, or neutral in nature. ant sting. Acids bases and salts cbse class 7 science revision notes chapter 5 . acids. an acid is a chemical substance that has a sour taste. many food items such as lemons, curd, vinegar and orange taste sour because of the presence of acid in them. acidic substances are the substances that contain acid in them. natural acids are the acids that occur in. Q.5.describe the process of neutralisation with the help of an example. ans.the reaction between an acid and a base is known as neutralisation. salt and water are produced in this process with the evolution of heat. antacids like milk of magnesia (magnesium hydroxide), baking soda, etc. which contain a base are used for reducing acidity in.

Comments are closed.