Ap Chemistry 8 2 Ph And Poh Of Strong Acids And Bases 2

Ph And Poh Scale Past papers. edexcel. spanish. past papers. cie. spanish language & literature. past papers. other subjects. revision notes on ph & poh of strong acids & bases for the college board ap chemistry syllabus, written by the chemistry experts at save my exams. Mr. mahan vodcast covering ap 8.2. in this vodcast i explain what makes a "strong acid" strong and what makes a "weak acid" weak; while discussing the impac.
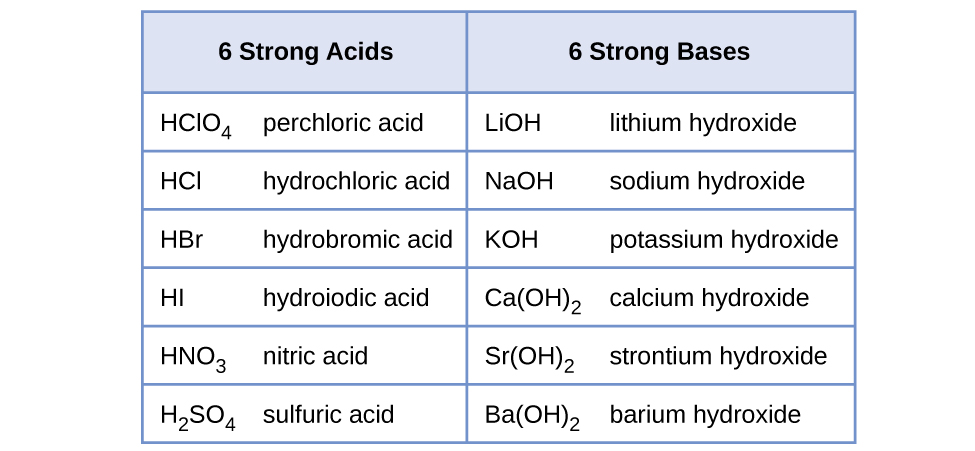
17 7 Relative Strengths Of Acids And Bases вђ Enhanced Introductory The same idea applies to strong bases. suppose we had 1m naoh. naoh > na oh , so poh = log([oh ]) = log(1) = 0. to find ph, we then plug our value into ph poh = 14, which tells us that the ph of a 1m solution of naoh is 14. the list of strong acids. there are seven strong acids you need to memorize for ap chemistry. In this video, mr. krug shows students how to calculate the ph of strong acids and bases. he works several problems, including a problem where two strong ac. By studying the ph and poh of strong acids and bases for the ap chemistry exam, you should be able to: understand the concept of complete dissociation in strong acids and bases; accurately calculate the hydrogen ion concentration [h⁺] and hydroxide ion concentration [oh⁻]; use these concentrations to determine the ph and poh of solutions. About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright.
Acids And Bases Mchs Science By studying the ph and poh of strong acids and bases for the ap chemistry exam, you should be able to: understand the concept of complete dissociation in strong acids and bases; accurately calculate the hydrogen ion concentration [h⁺] and hydroxide ion concentration [oh⁻]; use these concentrations to determine the ph and poh of solutions. About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright. Due to the acid fully ionizing, there is a high concentration of h h 3 o ions resulting in the solution having a low ph. the h h 3 o concentration is equal to the molar concentration of the acid: [h (aq)] = mstrong acid this can be used to calculate the ph using: ph = log 10 [h (aq)] the position of the equilibrium is so far over to. Unit 8.2: ph and poh of strong acids & bases (paired with 8.1) unit 8.3: weak acid and base equilibrium video 2. ap chemistry ap daily 8.4: video 3. ap chemistry.

How To Calculate The Ph Of A Strong Acid Solution Chemistry Study Due to the acid fully ionizing, there is a high concentration of h h 3 o ions resulting in the solution having a low ph. the h h 3 o concentration is equal to the molar concentration of the acid: [h (aq)] = mstrong acid this can be used to calculate the ph using: ph = log 10 [h (aq)] the position of the equilibrium is so far over to. Unit 8.2: ph and poh of strong acids & bases (paired with 8.1) unit 8.3: weak acid and base equilibrium video 2. ap chemistry ap daily 8.4: video 3. ap chemistry.

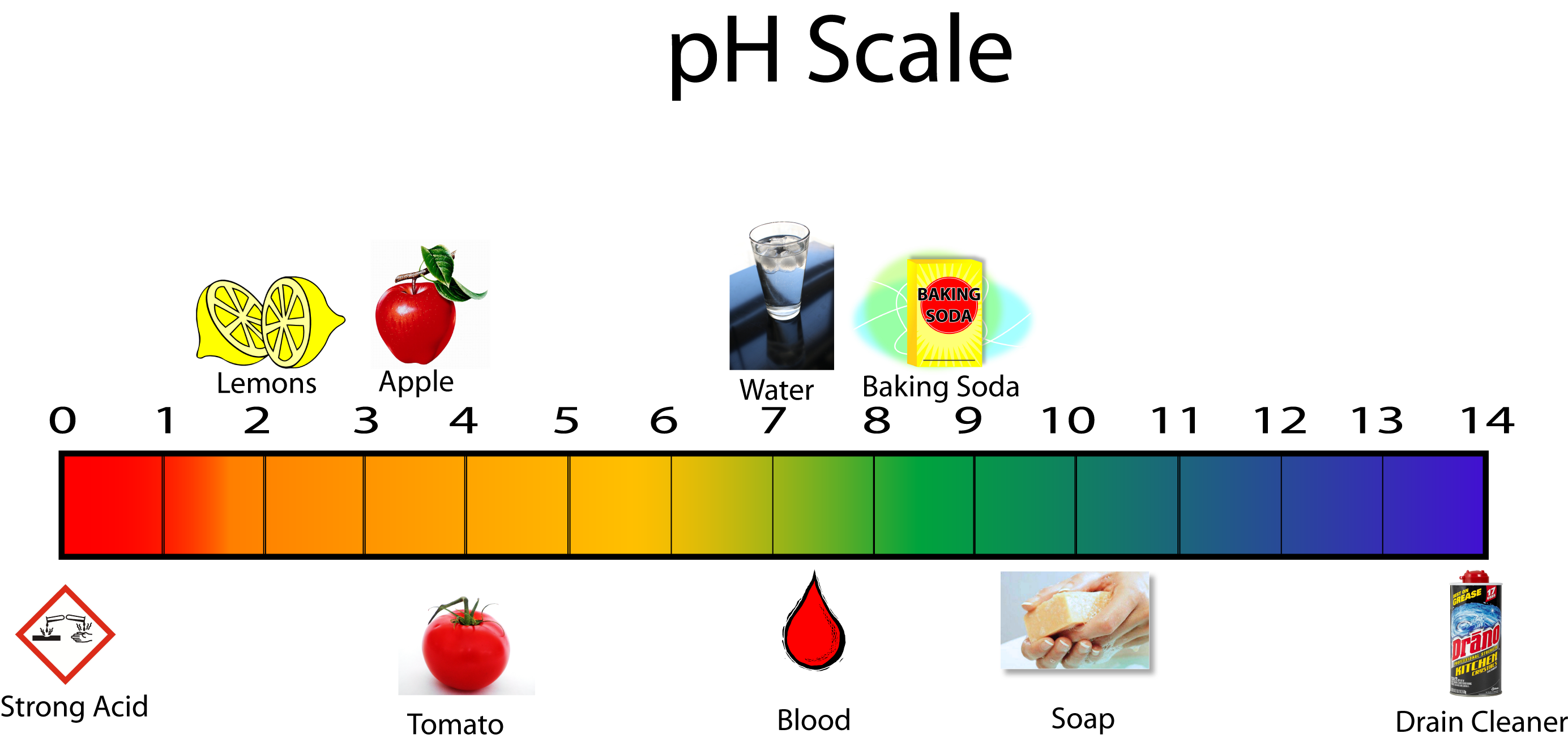
Know The Ph Scale

Comments are closed.