Basic Chemistry For Biology Part 4 Covalent Bonding And Structural
Basic Chemistry For Biology Part 4 Covalent Bonding And Structural This video series, basic chemistry for biology students, teaches the basic chemistry that you’ll need to know in your biology course, whether that’s introduc. Primary structure: the primary structure of a protein is its amino acid sequence. the base (repeat) sequence of the gene codes are comprised of three amino acids (glycine; proline; «x» any other amino acid). this sequence of amino acids bonded together creates a polypeptide (poly = many) bond, or chain.

Covalent Bond Biology Dictionary This structure is in part due to chemical interactions at work on the polypeptide chain. primarily, the interactions among r groups create the protein's complex three dimensional tertiary structure. the nature of the r groups in the amino acids involved can counteract forming the hydrogen bonds we described for standard secondary structures. Amino acid structure. amino acids are the monomers that make up proteins. each amino acid has the same core structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (nh2), a carboxyl group (cooh), and a hydrogen atom. every amino acid also has another atom or group of atoms bonded to the. The building blocks of proteins are amino acids, which are small organic molecules that consist of an alpha (central) carbon atom linked to an amino group, a carboxyl group, a hydrogen atom, and a. The shape of a protein is specified by its amino acid sequence. recall from chapter 2 that there are 20 types of amino acids in proteins, each with different chemical properties. a protein molecule is made from a long chain of these amino acids, each linked to its neighbor through a covalent peptide bond (figure 3 1).

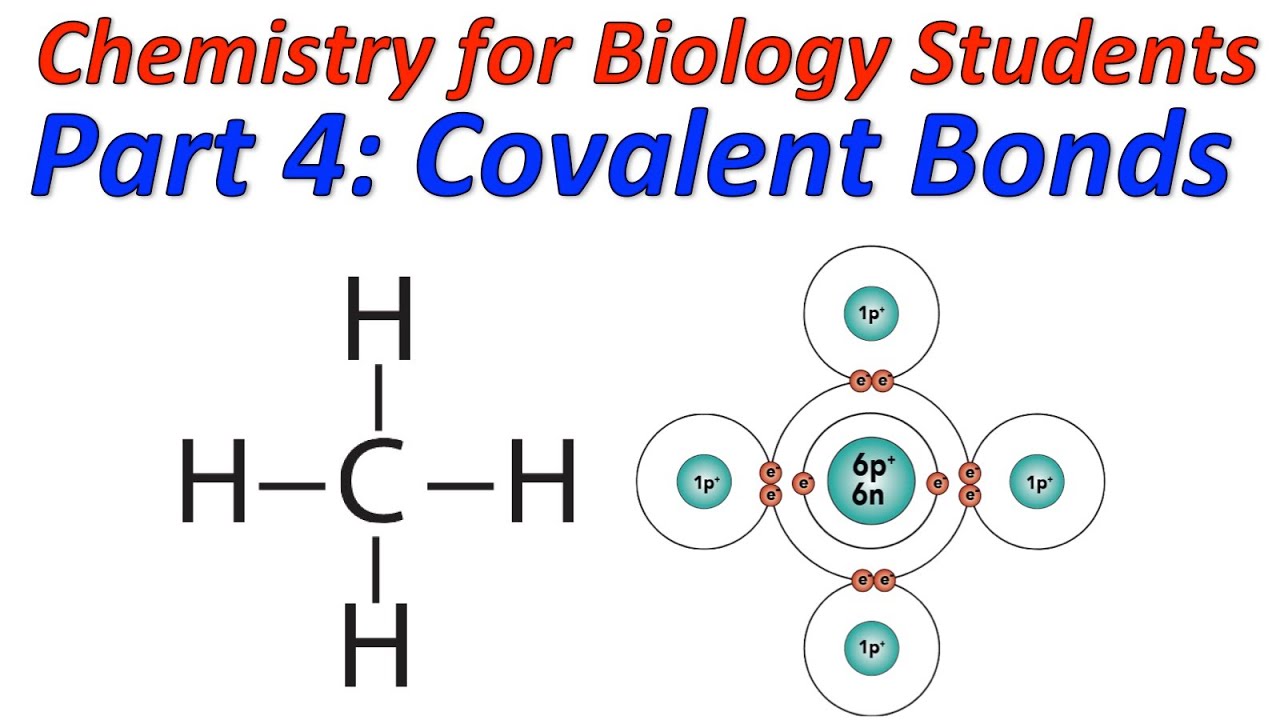
Covalent Bond Examples The building blocks of proteins are amino acids, which are small organic molecules that consist of an alpha (central) carbon atom linked to an amino group, a carboxyl group, a hydrogen atom, and a. The shape of a protein is specified by its amino acid sequence. recall from chapter 2 that there are 20 types of amino acids in proteins, each with different chemical properties. a protein molecule is made from a long chain of these amino acids, each linked to its neighbor through a covalent peptide bond (figure 3 1). A covalent bond is formed between two atoms by sharing electrons. the number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. hydrogen is an exception to the octet rule. h forms only one bond because it needs only two electrons. A peptide bond, scientifically referred to as an eupeptide bond, is a pivotal chemical bond formed by conjugating the carboxyl group of one amino acid to the amino group of another. this bond is characterized as an amide type of covalent chemical bond. it serves as a bridge linking two sequential alpha amino acids, specifically from c1 (carbon.

Comments are closed.