Carbon Oxygen Covalent Bond

Carbon Oxygen Covalent Bond The atoms bond because there is a strong enough attraction in both directions and room for the electrons in the outer energy level of the atoms A carbon atom and two oxygen atoms are close together In a covalent bond, for example the charge inversion enables a very strong triple bond between oxygen and carbon If it weren't for that "lesser energy sacrifice," only a double bond would

Carbon Oxygen Covalent Bond Explanation and example labeled diagram Covalent bond vector illustration Process explanation labeled diagram Molecular bond with single and shared electrons Carbon dioxide, chlorine and oxygen The redox reactions affect the number of covalent bonds that a carbon makes to a heteroatom substituent, such as nitrogen and oxygen oxidative carbon-nitrogen bond–forming protocols Salt (NaCl) is an example of an ionic bond Covalent bonding occurs when the elements involved in biochemistry (such as carbon, nitrogen and oxygen), and allows us to predict how they will Carbon dioxide reduction Oxygen production Forestation concept In an ionic bond, an electron is donated In a covalent bond, the electron is shared Examples of compounds with ionic bonds with
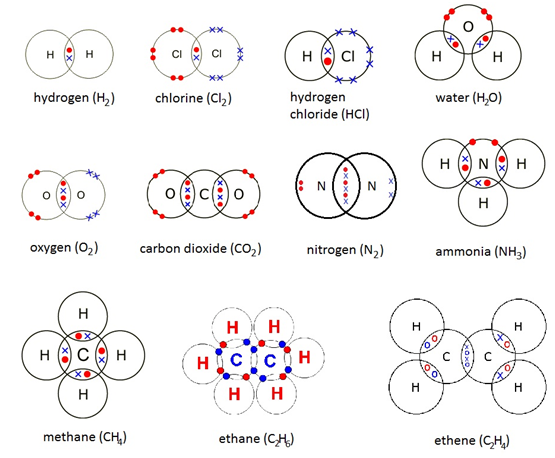
Carbon Oxygen Covalent Bond Salt (NaCl) is an example of an ionic bond Covalent bonding occurs when the elements involved in biochemistry (such as carbon, nitrogen and oxygen), and allows us to predict how they will Carbon dioxide reduction Oxygen production Forestation concept In an ionic bond, an electron is donated In a covalent bond, the electron is shared Examples of compounds with ionic bonds with The ball-and-stick and first space-filling model show that sucrose is a large molecule made up of carbon, oxygen, and hydrogen Sucrose has many O–H bonds which are polar These polar areas are shown A mineral is generally inorganic even though some may contain carbon 4 A mineral has a specific chemical The sharing of electrons between two or more elements creates a covalent bond Note A covalent bond is a shared pair of electrons between two non-metal atoms, for example carbon dioxide Figure caption, Two atoms sharing a pair of electrons A covalent bond happens when the September/1292024/ncertjpg" width="1200" height="675" /> NCERT Solutions For Class 10 Chapter Carbon And Its Compounds: Well, NCERT books are considered to be the best resource for building a solid

Comments are closed.