Cellular Senescence Its Role In Aging And Disease Process
Senescence In Physiological Processes And Age Related Diseases Cellular senescence plays a direct role in chronic and age related diseases and conditions, such as diabetes, atherosclerosis, neurovascular dysfunction, frailty, and dementias. accumulation of senescent cells with aging contributes to multiple, age related comorbidities that are frequently accompanied by neurodegenerative diseases, especially ad. Cellular senescence, a process that imposes permanent proliferative arrest on cells in response to various stressors, has emerged as a potentially important contributor to aging and age related disease, and it is an attractive target for therapeutic exploitation. a wealth of information about senescence in cultured cells has been acquired over.

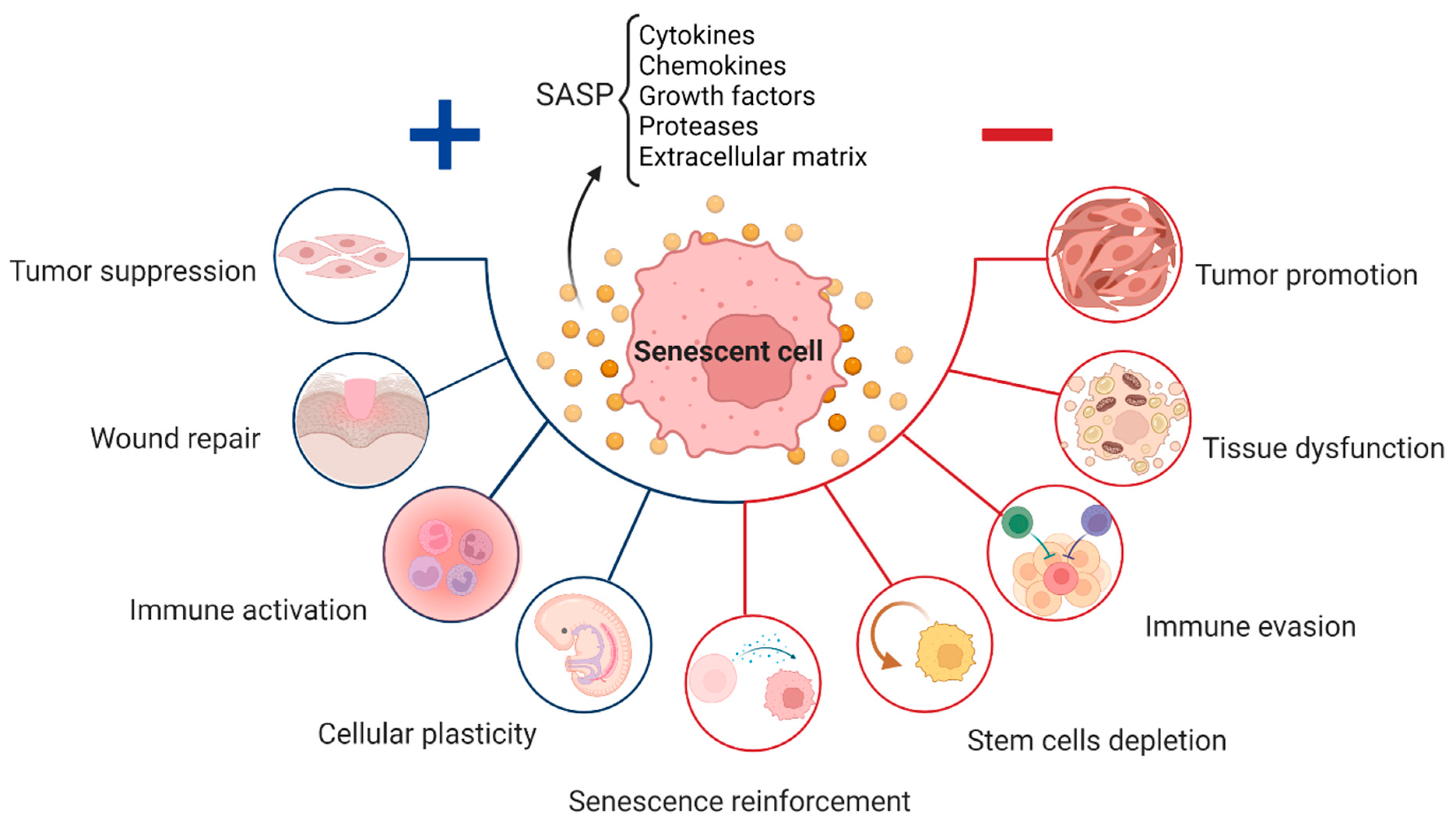
Cell Process How Is Cellular Senescence Related To Aging Cellular senescence is a hallmark of aging defined by stable exit from the cell cycle in response to cellular damage and stress. senescent cells (sncs) can develop a characteristic pathogenic senescence associated secretory phenotype (sasp) that drives secondary senescence and disrupts tissue homeostasis, resulting in loss of tissue repair and regeneration. Senescence as a central hallmark of aging. telomere damage, epigenetic dysregulation, dna damage, and mitochondrial dysfunction are primary drivers of damage in aging. several of these drivers of damage can induce senescence. senescence can in turn drive the consequential aging hallmarks in response to damage: stem cell exhaustion and chronic. Cellular senescence is a stable and terminal state of growth arrest in which cells are unable to proliferate despite optimal growth conditions and mitogenic stimuli (boxes 1,2; fig. 1).senescent. Abstract. cellular senescence represents a distinct cell fate characterized by replicative arrest in response to a host of extrinsic and intrinsic stresses. senescence facilitates programming.

The Role Of Senescence And Its Reversion In Healthy Aging And Cellular senescence is a stable and terminal state of growth arrest in which cells are unable to proliferate despite optimal growth conditions and mitogenic stimuli (boxes 1,2; fig. 1).senescent. Abstract. cellular senescence represents a distinct cell fate characterized by replicative arrest in response to a host of extrinsic and intrinsic stresses. senescence facilitates programming. The senescent cell became a major actor of the aging process, among others, by the acquisition of the senescence associated secretory phenotype. this chapter is devoted to the regulatory process involved in the acquisition of the senescent cell phenotype and its role in organismal aging. Aging is a complex process driven, at least in part, by hallmarks of aging, including cellular senescence, genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, stem cell exhaustion, and altered intercellular communication (1, 2). of these hallmarks, cellular senescence has been directly implicated as a.

Cellular Senescence Aging Cancer And Injury Physiological Reviews The senescent cell became a major actor of the aging process, among others, by the acquisition of the senescence associated secretory phenotype. this chapter is devoted to the regulatory process involved in the acquisition of the senescent cell phenotype and its role in organismal aging. Aging is a complex process driven, at least in part, by hallmarks of aging, including cellular senescence, genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, stem cell exhaustion, and altered intercellular communication (1, 2). of these hallmarks, cellular senescence has been directly implicated as a.

Comments are closed.