Chemical Reactions And Equations Class 10 Notes Cbse Science Chapte
Class 10 Science Chapter Notes Of Chemical Reactions And 4 A chemical equation can be divided into two types: balanced chemical equation and unbalanced chemical equation. (a) balanced chemical equation: a balanced chemical equation has the number of atoms of each element equal on both sides. example: zn h 2 so 4 → znso 4 h 2. Hydrogen ions gain electrons from the cathode and form hydrogen gas, and oxygen ions give electrons to the anode and form oxygen gas. chemical reactions and equations, class 10 chapter 1 science notes help students to study effectively and score higher marks in exams. these notes includes explanation for all the concepts provided in the chapter.
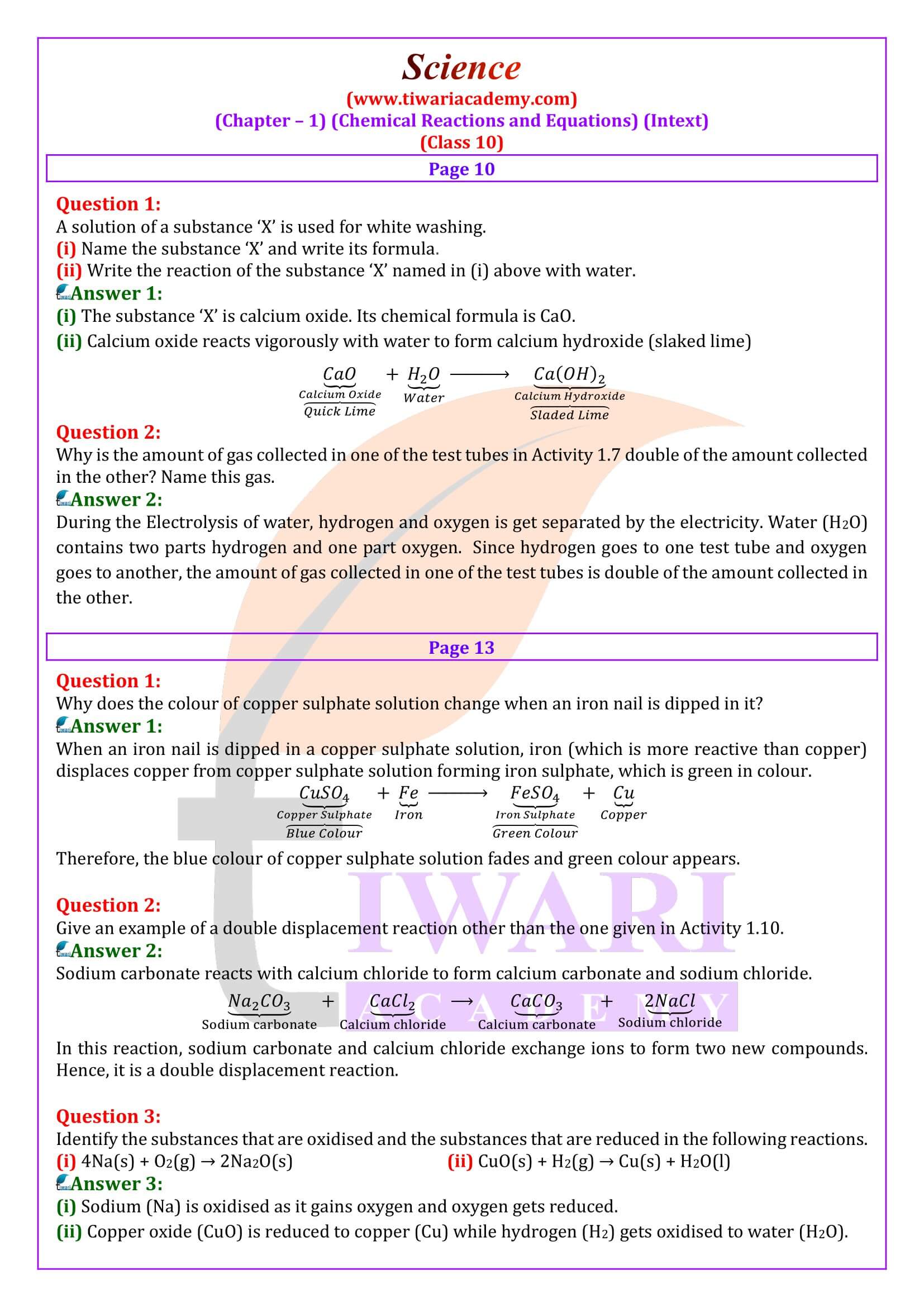
Class 10 Cbse Science Chapter 1 Chemical Reactions Equa Cbse class 10 science chapter wise notes. chapter 1 chemical reactions and equations notes. chapter 2 acids, bases and salts notes. chapter 3 metals and non metals notes. chapter 4 carbon and its compounds notes. chapter 5 periodic classification of elements notes. chapter 6 life processes notes. Here is a cbse class 10 science chapter 1 chemical reactions and equations notes and ncert solutions to important question answers. given here is the complete explanation of the chapter, along with examples and all the exercises, questions and answers are given at the back of the chapter. Cbse class 10 science notes chapter 1 chemical reaction and equation notes. below are some notes on cbse class 10 science, chapter 1: “chemical reactions and equations.” chemical reactions and equations 1. introduction: a chemical reaction involves the transformation of one or more substances into new substances. Page number: 10. question 1. a solution of a substance ‘x’ is used for white washing. (i) name the substance ‘x’ and write its formula. (ii) write the reaction of the substance ‘x’ named in (i) above with water. answer: (i) the substance whose solution in water is used for white washing is calcium oxide (or quick lime). its formula.

Class 10 Science Chapter 1 Notes Chemical Reactions And Cbse class 10 science notes chapter 1 chemical reaction and equation notes. below are some notes on cbse class 10 science, chapter 1: “chemical reactions and equations.” chemical reactions and equations 1. introduction: a chemical reaction involves the transformation of one or more substances into new substances. Page number: 10. question 1. a solution of a substance ‘x’ is used for white washing. (i) name the substance ‘x’ and write its formula. (ii) write the reaction of the substance ‘x’ named in (i) above with water. answer: (i) the substance whose solution in water is used for white washing is calcium oxide (or quick lime). its formula. Chapter 1 class 10 chemical reactions and equations. click on any of the links below to start learning from teachoo learn maths, science, gst and finance at teachoo. important. teachoo ad free version. maths 1 to 1 classes. skillistan. gst simulation portal. sample paper solutions. Chemical reaction and equations class 10 notes science chapter 1. chapter at a glance. chemical reaction: the process in which a substance undergoes change to produce new substances with new properties is known as a chemical reaction, e.g., magnesium carbonate when heated produces magnesium oxide and carbon dioxide (i.e. new substances with new.

Comments are closed.