Chemistry Essentials Whats The Atomic Number Of An Element
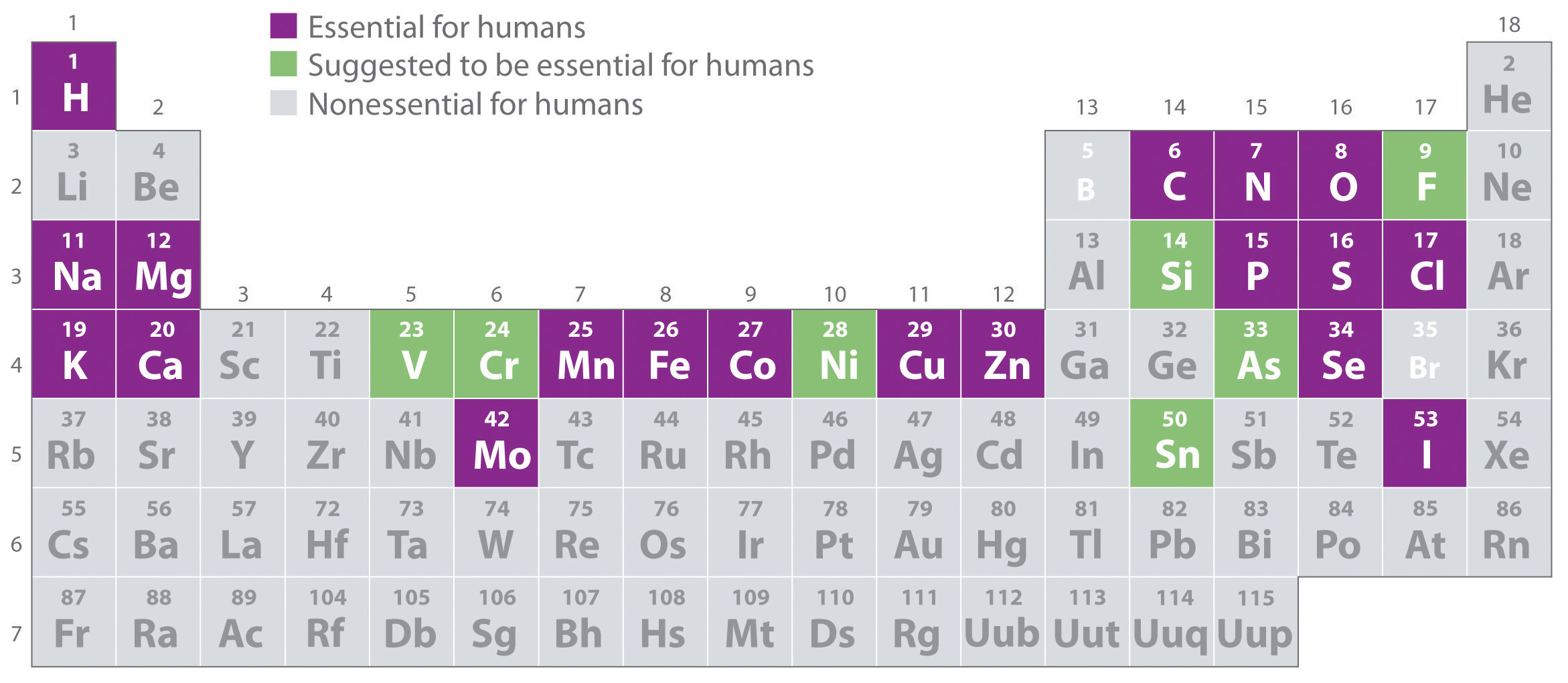
1 8 Essential Elements For Life Chemistry Libretexts This name describes the atomic number of the element, followed by the ium suffix. for example, element 120 has the temporary name of unbinilium. the temporary names are cumbersome, so it’s perfectly acceptable to refer to an unverified element by its atomic number (e.g., element 122, element 145). The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. the atomic number is also called the proton number. it is denoted by the symbol z and is the subscript in atomic notation. the symbol z comes from the german word zahl, which means numeral, or atomzahl, which means atomic number.
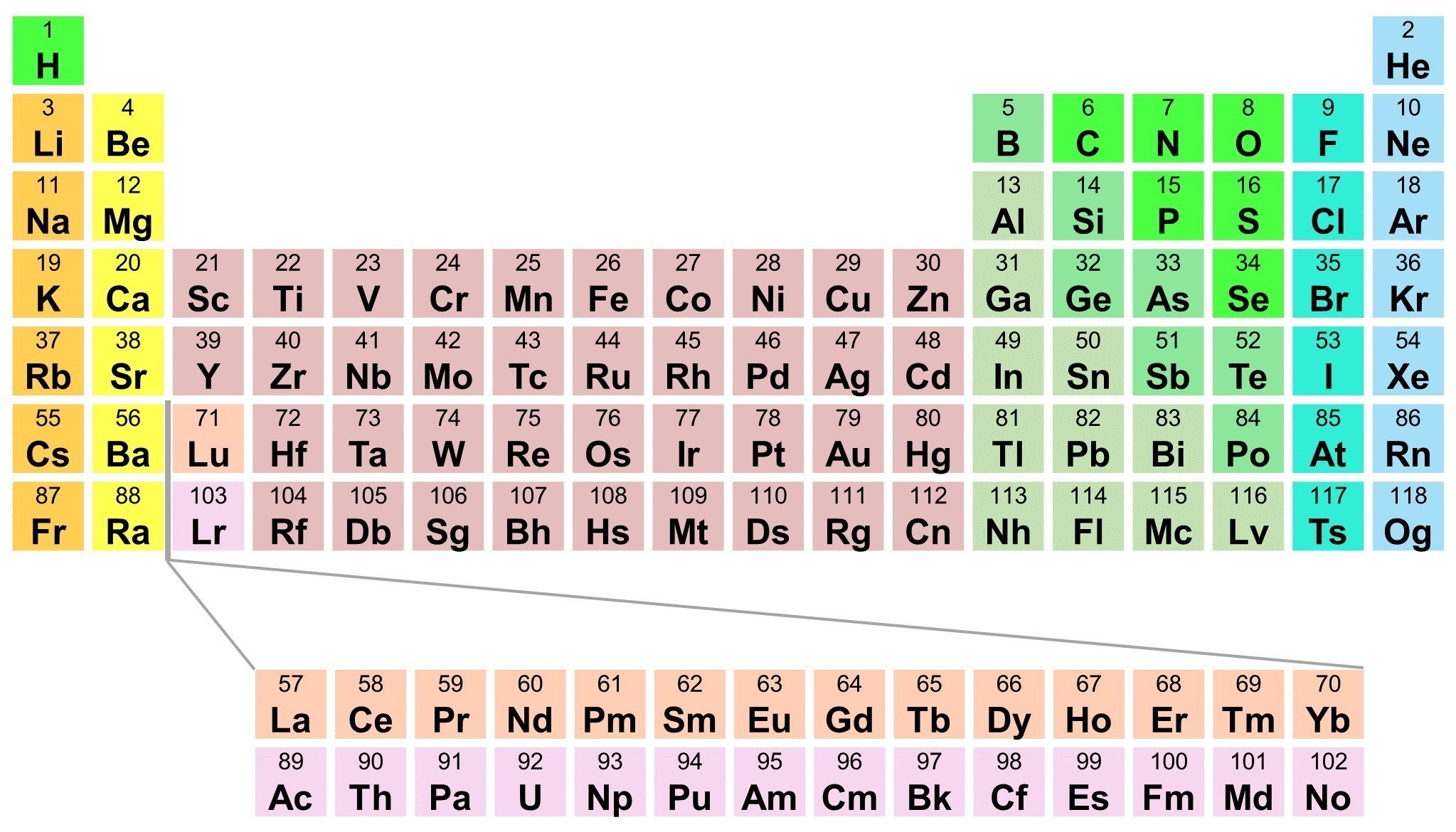
Periodic Table Of Elements With Names And Symbols And Atomic Mass And A quick definition of the atomic number of an element.chem fairy: louise mccartneydirector: michael harrisonwritten and produced by kimberly hatch harrison♦♦. The periodic table of elements is widely used in the field of chemistry to look up chemical elements as they are arranged in a manner that displays periodic trends in the chemical properties of the elements. however, the periodic table generally displays only the symbol of the element and not its entire name. 82. 12. 4.2: atomic number is shared under a not declared license and was authored, remixed, and or curated by libretexts. the modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. what makes atoms of different elements different? the fundamental …. The atomic number or nuclear charge number (symbol z) of a chemical element is the charge number of an atomic nucleus. for ordinary nuclei composed of protons and neutrons, this is equal to the proton number (np) or the number of protons found in the nucleus of every atom of that element. the atomic number can be used to uniquely identify.
/periodic-table-of-the-elements-2017--illustration-769723031-5ac10eb6a9d4f9003769784d.jpg)
Element List Atomic Number Element Name And Symbol 82. 12. 4.2: atomic number is shared under a not declared license and was authored, remixed, and or curated by libretexts. the modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. what makes atoms of different elements different? the fundamental …. The atomic number or nuclear charge number (symbol z) of a chemical element is the charge number of an atomic nucleus. for ordinary nuclei composed of protons and neutrons, this is equal to the proton number (np) or the number of protons found in the nucleus of every atom of that element. the atomic number can be used to uniquely identify. 4.5: chemical symbols and the atomic number is shared under a not declared license and was authored, remixed, and or curated by libretexts. scientists distinguish between different elements by counting the number of protons in the nucleus. since an atom of one element can be distinguished from an atom of another element by the number of …. Atomic numbers are often listed as a subscript on the left side of an element’s symbol (figure 2.4.1 2.4. 1 ). figure 2.4.1 2.4. 1: unlike protons, the number of neutrons is not absolutely fixed for most elements. atoms that have the same number of protons, and hence the same atomic number, but different numbers of neutrons are called isotopes.
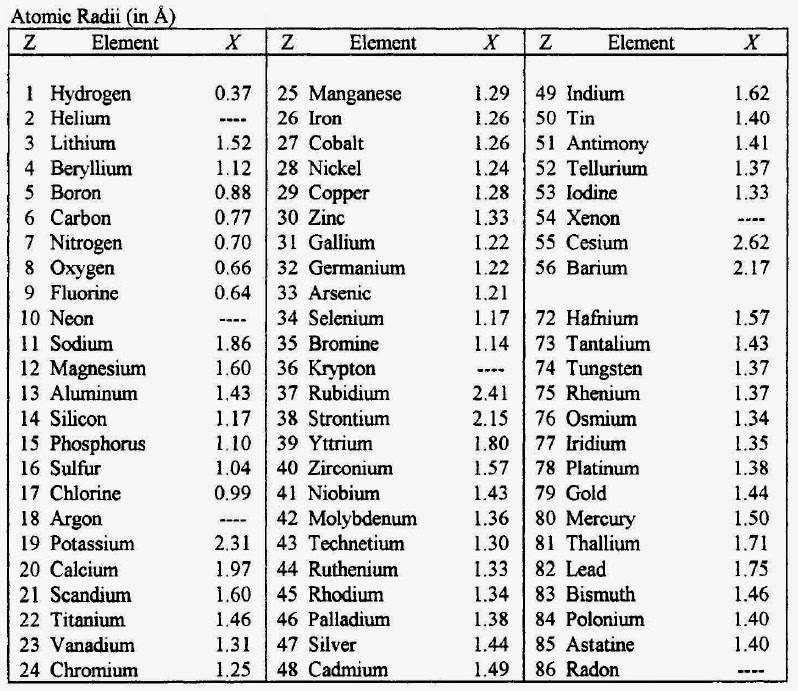
Printable Periodic Table With Atomic Number Dynamic Periodic Table Of 4.5: chemical symbols and the atomic number is shared under a not declared license and was authored, remixed, and or curated by libretexts. scientists distinguish between different elements by counting the number of protons in the nucleus. since an atom of one element can be distinguished from an atom of another element by the number of …. Atomic numbers are often listed as a subscript on the left side of an element’s symbol (figure 2.4.1 2.4. 1 ). figure 2.4.1 2.4. 1: unlike protons, the number of neutrons is not absolutely fixed for most elements. atoms that have the same number of protons, and hence the same atomic number, but different numbers of neutrons are called isotopes.

Comments are closed.