Class 10 Science Chemical Reactions And Equations Notes Important

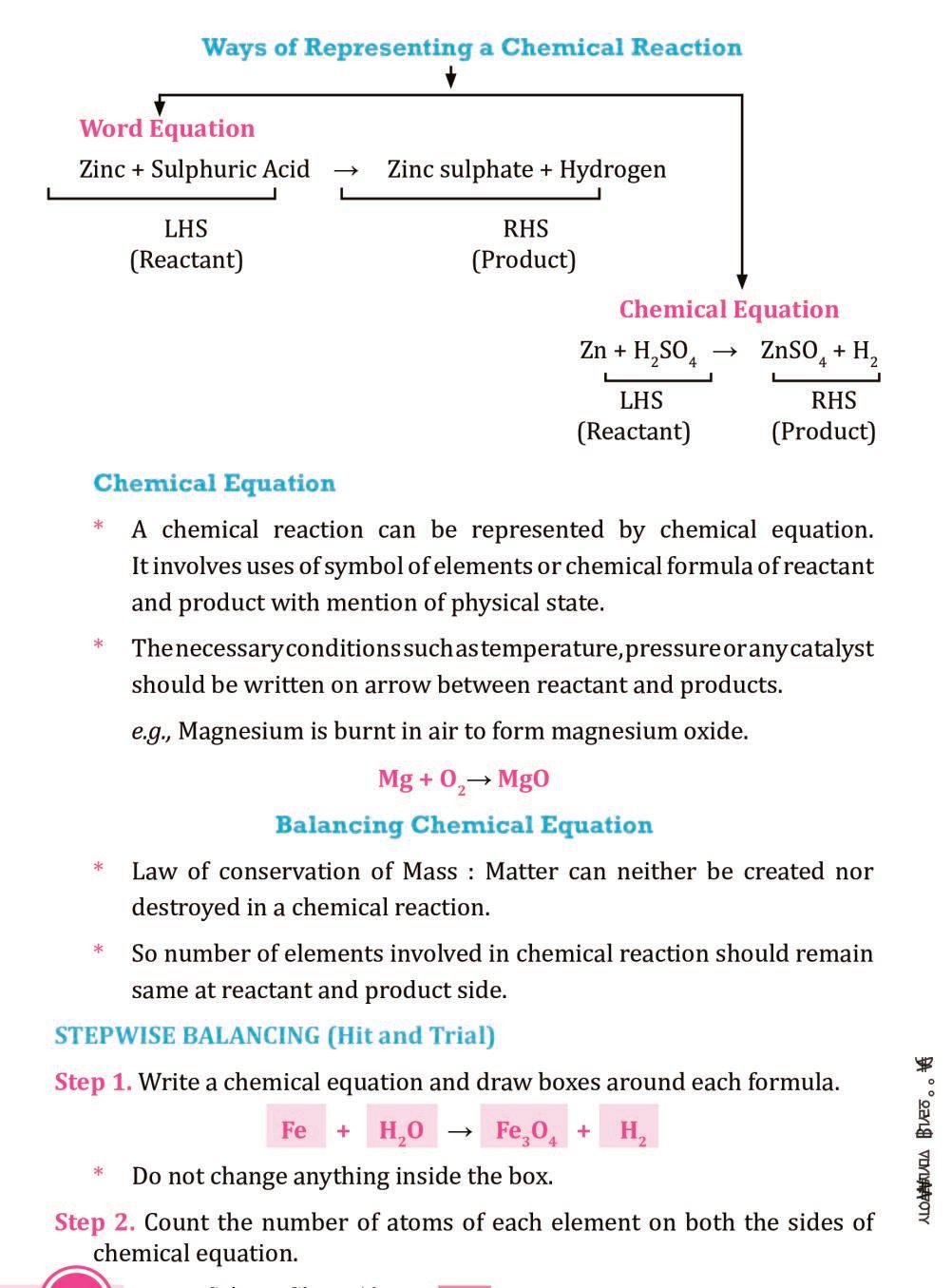
Class 10 Science Notes Chapter 1 Chemical Reactions And Equat A chemical equation can be divided into two types: balanced chemical equation and unbalanced chemical equation. (a) balanced chemical equation: a balanced chemical equation has the number of atoms of each element equal on both sides. example: zn h 2 so 4 → znso 4 h 2. Hydrogen ions gain electrons from the cathode and form hydrogen gas, and oxygen ions give electrons to the anode and form oxygen gas. chemical reactions and equations, class 10 chapter 1 science notes help students to study effectively and score higher marks in exams. these notes includes explanation for all the concepts provided in the chapter.

Class 10 Science Chemical Reactions And Equations Notes вђ The special name of this reaction is respiration is an exothermic reaction. c 6 h 12 o 6 (aq) 6o 2 (aq) → 6co 2 6h 2 o (l) energy. (glucose) → endothermic reaction: an endothermic process absorbs heat and cools the surroundings. the decomposition of vegetable matter into compost is also an example of an endothermic reaction. Cbse class 10 science chapter wise notes. chapter 1 chemical reactions and equations notes. chapter 2 acids, bases and salts notes. chapter 3 metals and non metals notes. chapter 4 carbon and its compounds notes. chapter 5 periodic classification of elements notes. chapter 6 life processes notes. The chemical reactions and equations notes here help you solve the questions and answers. also, you can complete the class 10 chemical reactions and equations worksheet using the same. in addition you will also tackle cbse class 10 science important questions with these class 10 notes. however if you still need help, then you can use the ncert. Here is a cbse class 10 science chapter 1 chemical reactions and equations notes and ncert solutions to important question answers. given here is the complete explanation of the chapter, along with examples and all the exercises, questions and answers are given at the back of the chapter.

Class 10th Science Chemicals Reactions And Equations Ncert No The chemical reactions and equations notes here help you solve the questions and answers. also, you can complete the class 10 chemical reactions and equations worksheet using the same. in addition you will also tackle cbse class 10 science important questions with these class 10 notes. however if you still need help, then you can use the ncert. Here is a cbse class 10 science chapter 1 chemical reactions and equations notes and ncert solutions to important question answers. given here is the complete explanation of the chapter, along with examples and all the exercises, questions and answers are given at the back of the chapter. Revision notesclass 10 sciencechapter 1 chemical reactions and equations chemical. change: a change that results in the formation of one. al changes are also known as chemical reactions. in a. hemical change— new substan. s are produced during a chemical reaction.changes. n energy are involved.during t. The ncert class 10 science chapter 1 notes give you a basic idea of the chapter chemical reactions and equations. the main topics covered in chapter 1 ncert class 10 science notes are definitions, examples, chemical equations and how to balance them, types of chemical reactions, the effects of oxidation reactions in everyday life.
Cbse Notes Class 10 Science Chemical Reactions And Equationsо Revision notesclass 10 sciencechapter 1 chemical reactions and equations chemical. change: a change that results in the formation of one. al changes are also known as chemical reactions. in a. hemical change— new substan. s are produced during a chemical reaction.changes. n energy are involved.during t. The ncert class 10 science chapter 1 notes give you a basic idea of the chapter chemical reactions and equations. the main topics covered in chapter 1 ncert class 10 science notes are definitions, examples, chemical equations and how to balance them, types of chemical reactions, the effects of oxidation reactions in everyday life.

Comments are closed.