Concentration Vs Solubility Meaning And Differences

Concentration Vs Solubility Meaning And Differences The difference between concentration and solubility is their nature. concentration is the amount of solute dissolved in a unit quantity of solution; solubility is the maximum amount of solute that can dissolve in a given amount of solvent under specific conditions (usually temperature and pressure). Concentration refers to the amount of solute present in a given amount of solvent or solution, while solubility describes the ability of a solute to dissolve in a particular solvent. concentration influences the properties and behavior of a solution, affects reaction rates, and plays a crucial role in analytical chemistry.
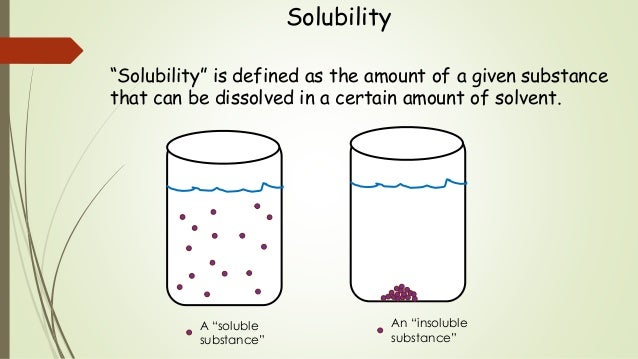
Solubility This chemistry video tutorial provides a basic introduction into solubility. it explains the difference between concentration and solubility. solubility re. Concentration solubility; definition: concentration refers to the amount of solute dissolved in a given amount of solvent at any specific concentration in a solution. solubility refers to the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature before reaching its saturation point. units. The solubility of a specific solute in a specific solvent is generally expressed as the concentration of a saturated solution of the two. [1] any of the several ways of expressing concentration of solutions can be used, such as the mass, volume, or amount in moles of the solute for a specific mass, volume, or mole amount of the solvent or of the solution. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution process is at equilibrium. referring to the example of salt in water: nacl(s) ⇌ na (aq) cl−(aq) nacl (s) ⇌ na (a q) cl − (a q). when a solute’s concentration is equal to its solubility.

Solubility And Concentration Worksheet The solubility of a specific solute in a specific solvent is generally expressed as the concentration of a saturated solution of the two. [1] any of the several ways of expressing concentration of solutions can be used, such as the mass, volume, or amount in moles of the solute for a specific mass, volume, or mole amount of the solvent or of the solution. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution process is at equilibrium. referring to the example of salt in water: nacl(s) ⇌ na (aq) cl−(aq) nacl (s) ⇌ na (a q) cl − (a q). when a solute’s concentration is equal to its solubility. Solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as grams of solute per litre of solvent). solubility of one fluid (liquid or gas) in another may be complete (totally miscible; e.g., methanol and water) or partial (oil and water dissolve only. According to henry’s law, for an ideal solution the solubility, cg, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly proportional to the pressure, pg, of the undissolved gas above the solution (101.3 kpa in this case). because both cg and pg are known, this relation can be rearranged and used to solve for k.

Comments are closed.