Cooling Curve Phase Diagram

Heating And Cooling Curves вђ Overview Examples Expii By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa. A cooling curve for a sample that begins at the temperature and composition given by point a is shown in figure 8.10.1b 8.10. 1 b. figure 8.10.1 8.10. 1: (a) cooling of a two component system from liquid to solid. (b) cooresponding cooling curve for this process. as the sample cools from point a, the temperature will decrease at a rate.

Digging Into Phase Diagrams Cooling Curves Physical Chemistry Using the phase diagram. suppose you have a mixture of 67% lead and 33% tin. that's the mixture from the first cooling curve plotted above. suppose it is at a temperature of 300°c. that corresponds to a set of conditions in the area of the phase diagram labeled as molten tin and lead. now consider what happens if you cool that mixture. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is. The cooling curve phase diagram is a graphical representation of this solidification process, which helps us understand the behavior of different substances during solidification. the cooling curve phase diagram consists of two axes: temperature and time. it shows the temperature of the substance on the y axis and the time on the x axis. The cooling curve method is one of the oldest and simplest methods to determine phase diagrams and phase transition temperatures. this is achieved by recording temperature (t) of a material versus time as it cools from its molten state through solidification (at constant pressure). whenever a phase change takes place in a metal or alloy, the.
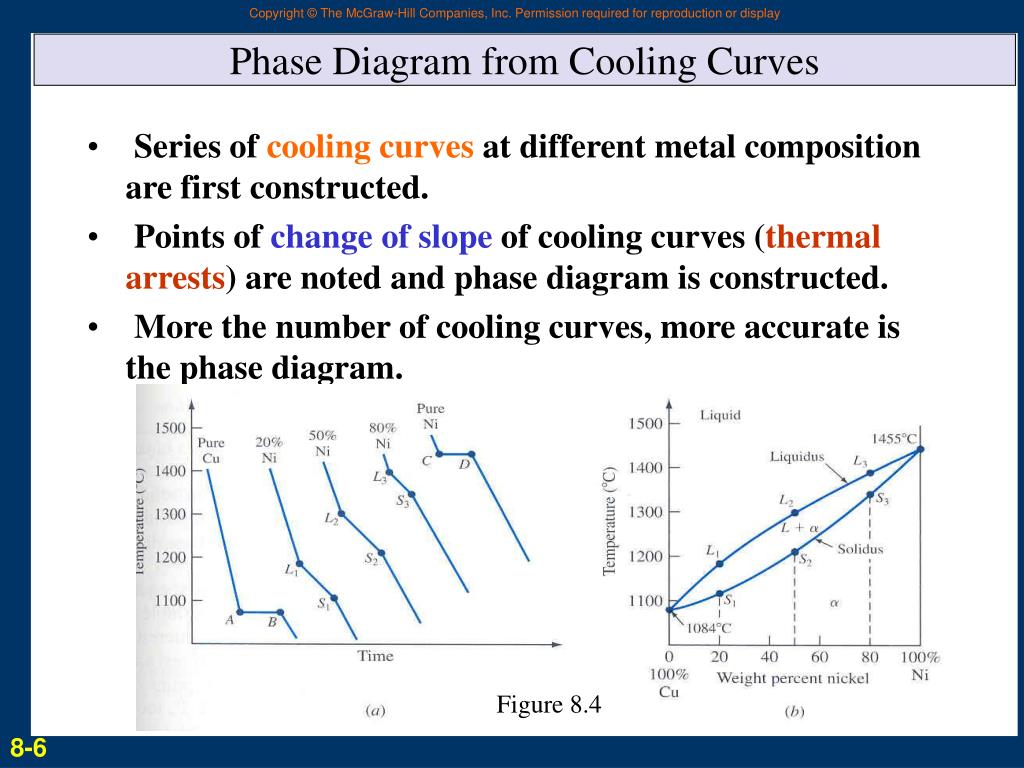
Cooling Curve Phase Diagram The cooling curve phase diagram is a graphical representation of this solidification process, which helps us understand the behavior of different substances during solidification. the cooling curve phase diagram consists of two axes: temperature and time. it shows the temperature of the substance on the y axis and the time on the x axis. The cooling curve method is one of the oldest and simplest methods to determine phase diagrams and phase transition temperatures. this is achieved by recording temperature (t) of a material versus time as it cools from its molten state through solidification (at constant pressure). whenever a phase change takes place in a metal or alloy, the. As we increase the temperature, the pressure of the water vapor increases, as described by the liquid gas curve in the phase diagram for water (figure 10.31), and a two phase equilibrium of liquid and gaseous phases remains. at a temperature of 374 °c, the vapor pressure has risen to 218 atm, and any further increase in temperature results in. Organized by textbook: learncheme uses the information in a phase diagram to draw the temperature dependence on time as a binary liquid alloy is.
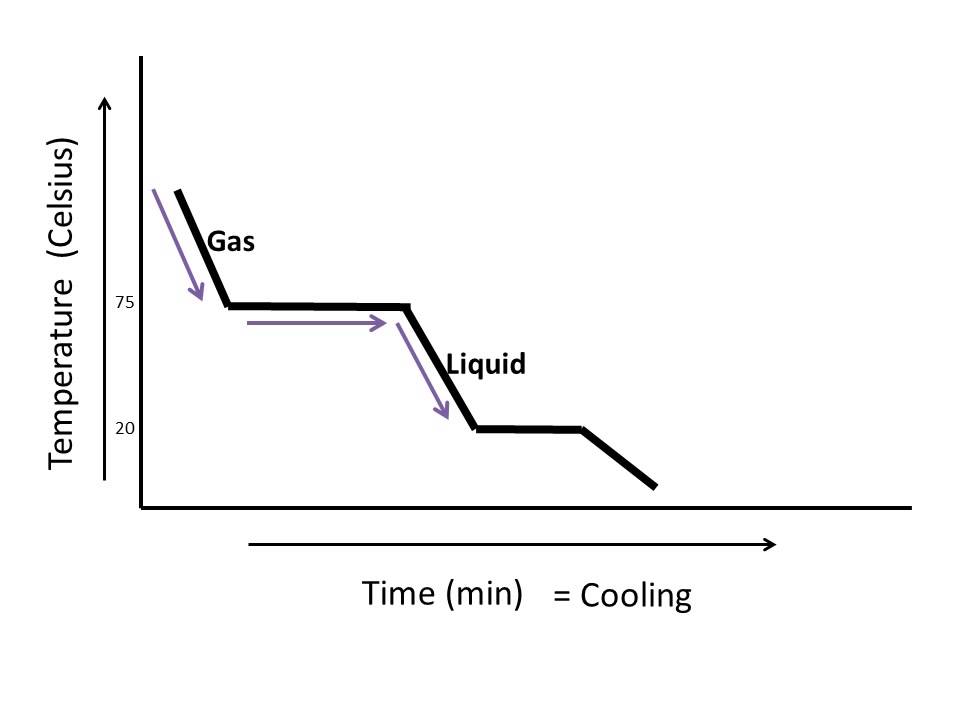
Heating And Cooling Curves Explained As we increase the temperature, the pressure of the water vapor increases, as described by the liquid gas curve in the phase diagram for water (figure 10.31), and a two phase equilibrium of liquid and gaseous phases remains. at a temperature of 374 °c, the vapor pressure has risen to 218 atm, and any further increase in temperature results in. Organized by textbook: learncheme uses the information in a phase diagram to draw the temperature dependence on time as a binary liquid alloy is.

Comments are closed.