Critical Point Phase Diagram

Critical Point Chemistry Dictionary Glossary Overview. the liquid–vapor critical point in a pressure–temperature phase diagram is at the high temperature extreme of the liquid–gas phase boundary. the dashed green line shows the anomalous behavior of water. Learn what the critical point is, the temperature and pressure at which liquid and gas cannot be distinguished, and how it affects the phase diagram of a substance. see problems and solutions involving the critical point and the lower critical solution temperature of polymers.
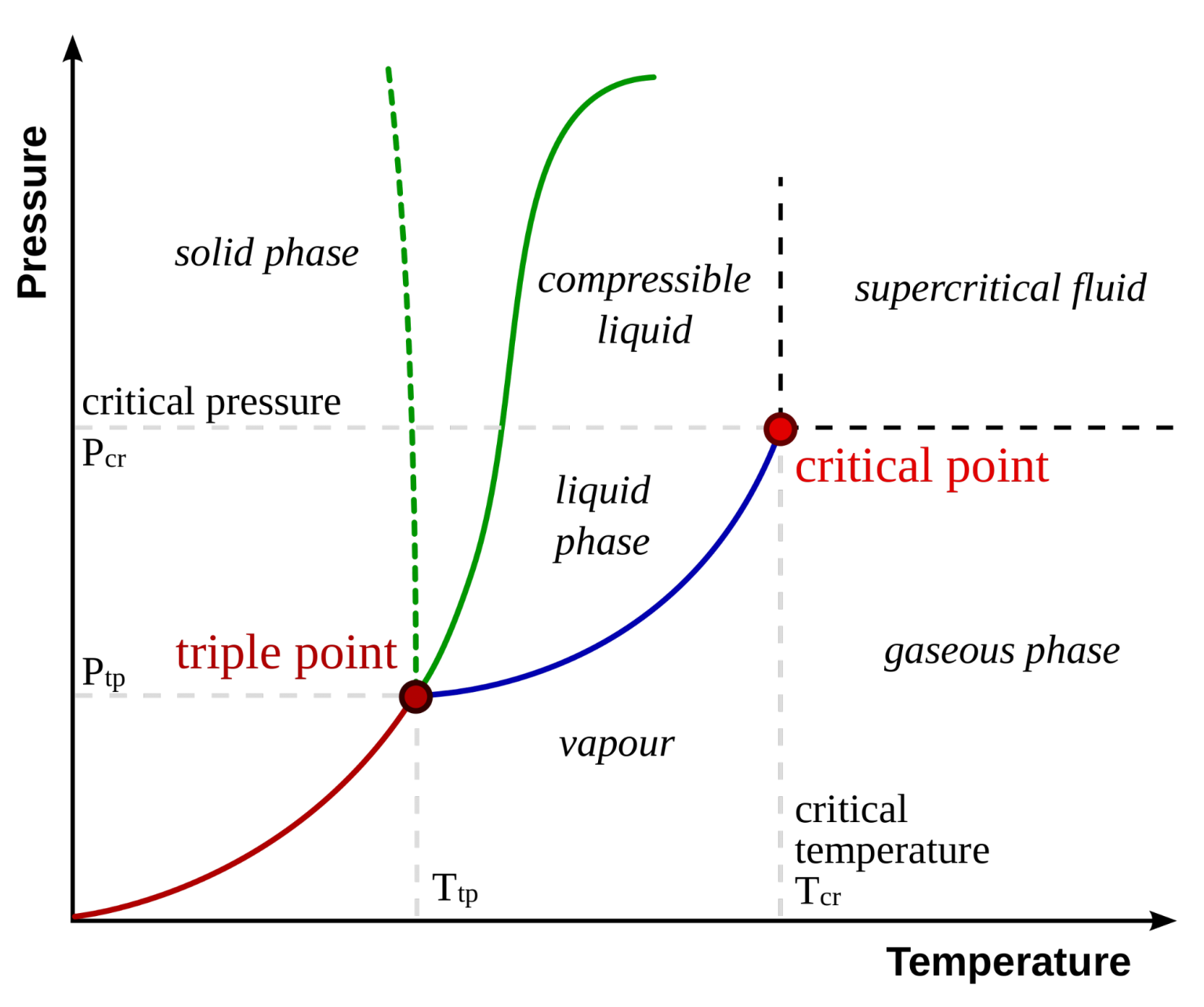
Critical Point Definition Critical Temperature Application The critical point. you will have noticed that this liquid vapor equilibrium curve has a top limit (labeled as c in the phase diagram), which is known as the critical point. the temperature and pressure corresponding to this are known as the critical temperature and critical pressure. Learn about phase diagrams, which graphically represent the physical states of a substance under different conditions of temperature and pressure. find out the definitions and examples of phase transitions, triple point, critical point, and sublimation. Critical point, in physics, the set of conditions under which a liquid and its vapour become identical (see phase diagram). for each substance, the conditions defining the critical point are the critical temperature, the critical pressure, and the critical density. this is best understood by observing a simple experiment. This free textbook is an openstax resource written to increase student access to high quality, peer reviewed learning materials.

Phase Change Diagrams вђ Overview Examples Expii Critical point, in physics, the set of conditions under which a liquid and its vapour become identical (see phase diagram). for each substance, the conditions defining the critical point are the critical temperature, the critical pressure, and the critical density. this is best understood by observing a simple experiment. This free textbook is an openstax resource written to increase student access to high quality, peer reviewed learning materials. A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. overview. []. Point c is the critical point of the substance, which is the highest temperature and pressure at which a gas and a liquid can coexist at equilibrium. the figure below shows what happens when we draw a horizontal line across a phase diagram at a pressure of exactly 1 atm.

Thermodynamics Behavior Beyond The Critical Pressure Chemistry A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. overview. []. Point c is the critical point of the substance, which is the highest temperature and pressure at which a gas and a liquid can coexist at equilibrium. the figure below shows what happens when we draw a horizontal line across a phase diagram at a pressure of exactly 1 atm.
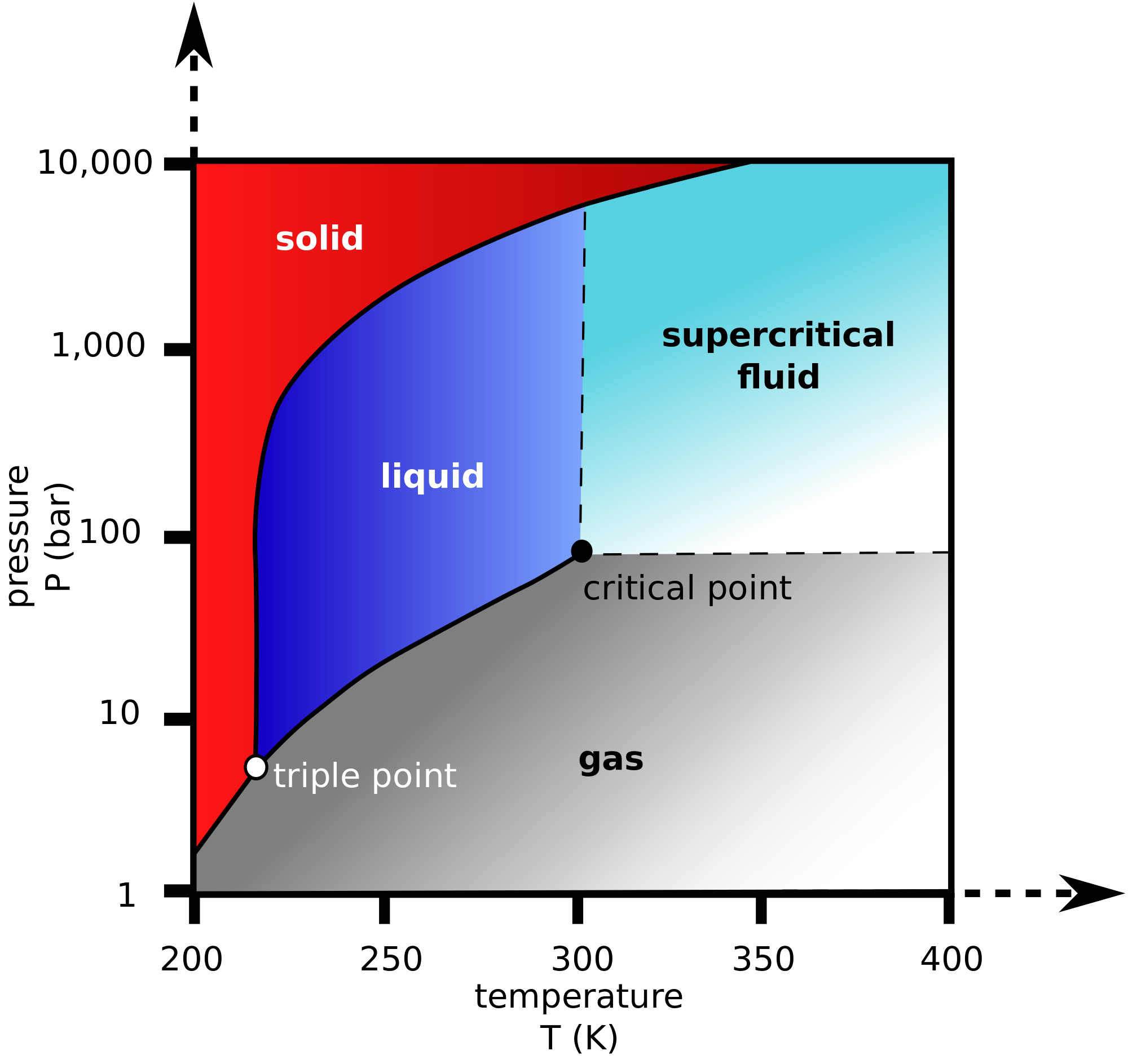
What Is The Relation Between Critical Temperature And Boiling Point Or

Comments are closed.