Demonstration Of Emission Spectra

Demonstration Of Emission Spectra Youtube Demonstration of emission spectra.chemistry lecture #24for a pdf transcript of this lecture, go to richardlouie. This is a demonstration of the continuous spectrum of white light and the emission spectra of mercury, nitrogen, neon, and hydrogen, imaged through a diffrac.

Emission Spectra Stars At Michael Ibarra Blog View emission spectra from a variety of light sources. stunning!this video is part of the flinn scientific best practices for teaching chemistry video series. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. when hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. however, when separated using a prism or diffraction grating, the. Objectives. to perform flame tests of metal cations in order to observe their characteristic colors, to perform calculations to determine the frequency and energy of the emitted photons. to relate these results to the types of electronic transitions occurring in these elements. to observe and understand line emission spectra of atoms using gas. This demonstration experiment can be used to show the flame colours given by alkali metal, alkaline earth metal, and other metal, salts. this is a spectacular version of the ‘flame tests’ experiment that can be used with chemists and non chemists alike. it can be extended as an introduction to atomic spectra for post 16 students.
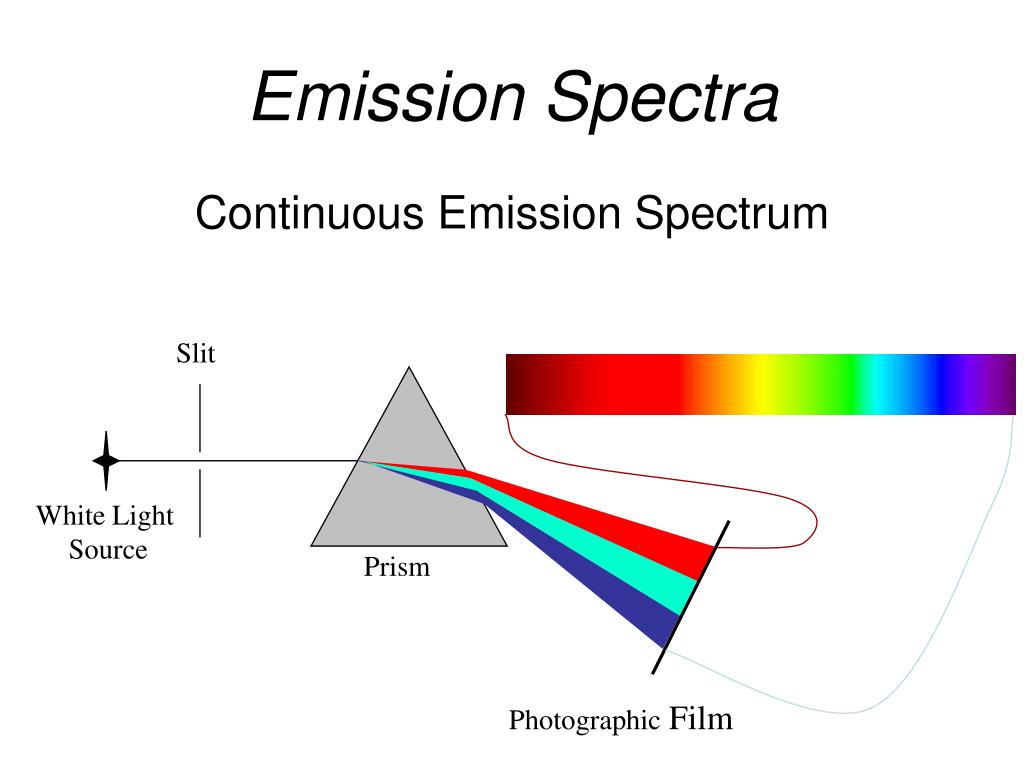
Ppt Properties Of Light Powerpoint Presentation Free Download Id Objectives. to perform flame tests of metal cations in order to observe their characteristic colors, to perform calculations to determine the frequency and energy of the emitted photons. to relate these results to the types of electronic transitions occurring in these elements. to observe and understand line emission spectra of atoms using gas. This demonstration experiment can be used to show the flame colours given by alkali metal, alkaline earth metal, and other metal, salts. this is a spectacular version of the ‘flame tests’ experiment that can be used with chemists and non chemists alike. it can be extended as an introduction to atomic spectra for post 16 students. Colored flames illustrate emission spectra and raise interest in science. people have been coloring flames pretty much since the days of prometheus. even before chemistry was recognized as a science, colored fire was a useful chemistry demonstration. E = hν (14a.2) (14a.2) e = h ν. these two relationships combine to give a third: e = hc λ (14a.3) (14a.3) e = h c λ. thus, the spectrum of an element can be stated by listing the particular wavelengths of light that its atoms emit. to measure these wavelengths in the laboratory, we must first separate them.

Comments are closed.