Different Properties Of Types Of Matter
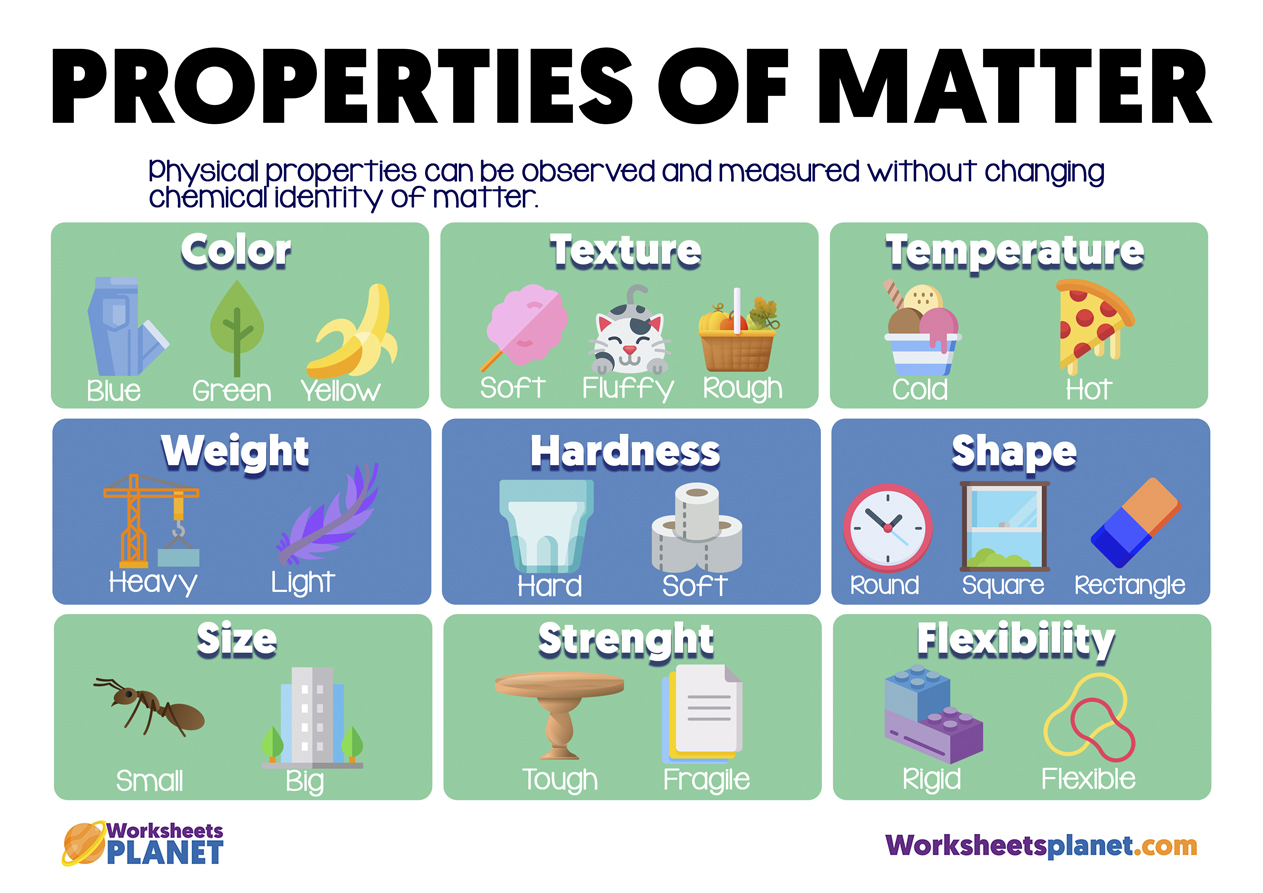
Different Properties Of Types Of Matter A physical property is an attribute of matter that is independent of its chemical composition. density, colour, hardness, melting and boiling points, and electrical conductivity are all examples of physical properties. any characteristic that can be measured, such as an object’s density, colour, mass, volume, length, malleability, melting. Chemical properties are characteristics that describe how matter changes its chemical structure or composition. an example of a chemical property is flammability—a material’s ability to burn—because burning (also known as combustion) changes the chemical composition of a material. oxidation, rusting, decomposition, and inertness are.
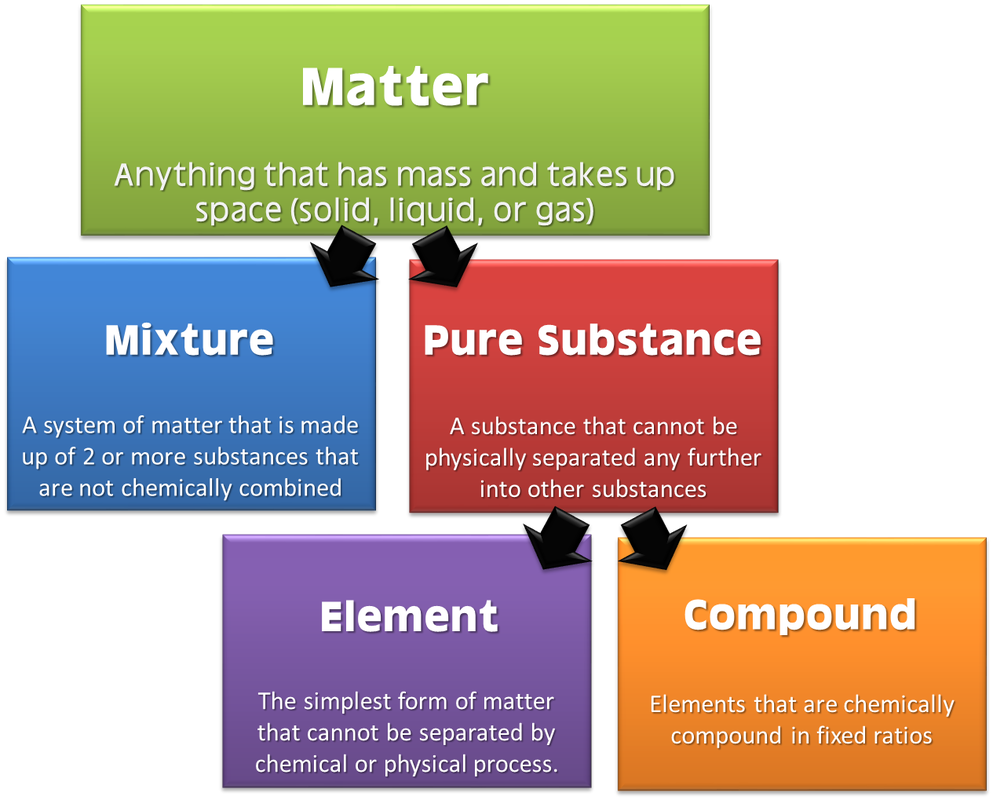
Matter Classification Properties And Changes Carpe Diem Figure 1.3.1 1.3. 1: the difference between extensive and intensive properties of matter. because they differ in size, the two samples of sulfur have different extensive properties, such as mass and volume. in contrast, their intensive properties, including color, melting point, and electrical conductivity, are identical. The ability to change from one type of matter into another (or the inability to change) is a chemical property. examples of chemical properties include flammability, toxicity, acidity, and many other types of reactivity. iron, for example, combines with oxygen in the presence of water to form rust; chromium does not oxidize (figure 1.19. Matter, material substance that constitutes the observable universe and, together with energy, forms the basis of all objective phenomena. at the most fundamental level, matter is composed of elementary particles known as quarks and leptons (the class of elementary particles that includes electrons). quarks combine into protons and neutrons and. A physical change is a change in the state or properties of matter without any accompanying change in its chemical composition (the identities of the substances contained in the matter). we observe a physical change when wax melts, when sugar dissolves in coffee, and when steam condenses into liquid water (figure 2.2.1 2.2. 1).

Comments are closed.