Digging Into Phase Diagrams Cooling Curves Physical Chemistry
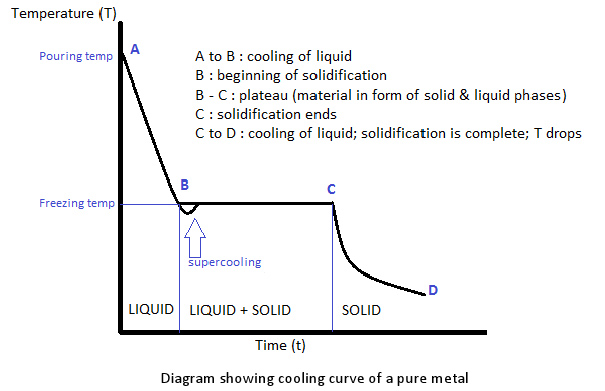
Digging Into Phase Diagrams Cooling Curves Physical Chemistry The cooling curve method is one of the oldest and simplest methods to determine phase diagrams and phase transition temperatures. this is achieved by recording temperature (t) of a material versus time as it cools from its molten state through solidification (at constant pressure). whenever a phase change takes place in a metal or alloy, the. A cooling curve for a sample that begins at the temperature and composition given by point a is shown in figure 8.10.1b 8.10. 1 b. figure 8.10.1 8.10. 1: (a) cooling of a two component system from liquid to solid. (b) cooresponding cooling curve for this process. as the sample cools from point a, the temperature will decrease at a rate.

Digging Into Phase Diagrams Cooling Curves Physical Chemistry By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling. 13.2.2 solid–liquid systems. figure 13.1 temperature–composition phase diagram for a binary system exhibiting a eutectic point. figure 13.1 is a temperature–composition phase diagram at a fixed pressure. the composition variable is the mole fraction of component b in the system as a whole. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is.
Comments are closed.