Examples Of Chemical And Physical Properties

Examples Of Chemical And Physical Properties Here is a list of several examples of chemical and physical properties. examples of chemical properties. in order to observe a chemical property, the chemical composition of a sample must be changed by a chemical process or reaction. flammability; toxicity; enthalpy of formation; heat of combustion; oxidation states; ph; half life; coordination. A physical property is a characteristic of a substance that can be observed or measured without changing the identity of the substance. physical properties include color, density, hardness, and melting and boiling points. a chemical property describes the ability of a substance to undergo a specific chemical change.
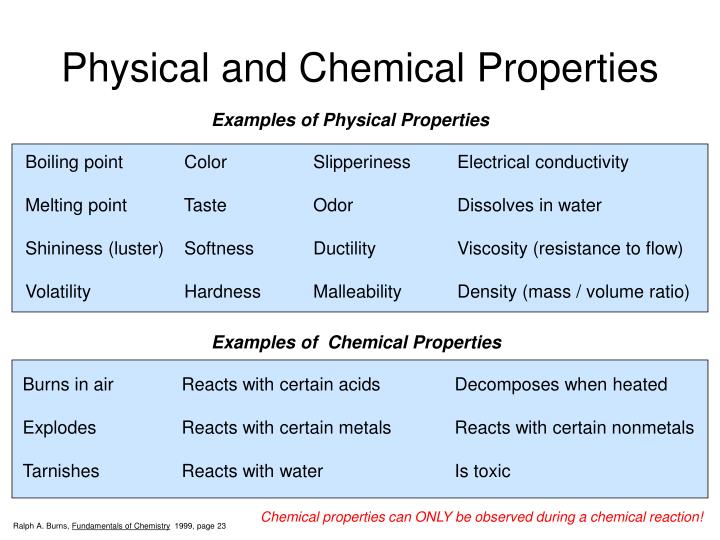
List Of Physical Properties In Chemistry The ability to change from one type of matter into another (or the inability to change) is a chemical property. examples of chemical properties include flammability, toxicity, acidity, and many other types of reactivity. iron, for example, combines with oxygen in the presence of water to form rust; chromium does not oxidize (figure 1.19. In other words, you have to change the chemical identity of a substance or rearrange its internal structure to know its chemical properties. in contrast, a physical property is observable and measurable without changing the internal structure of matter. examples of chemical properties. matter has many chemical properties. examples include. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. we can observe some physical properties, such as density and color, without changing the physical. Physical properties close physical property a property of an element or compound which can be directly observed or measured. for example, melting point, electrical conductivity, appearance at room.

Ppt Physical And Chemical Properties Powerpoint Presentation Free A physical property is a characteristic of matter that is not associated with a change in its chemical composition. familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. we can observe some physical properties, such as density and color, without changing the physical. Physical properties close physical property a property of an element or compound which can be directly observed or measured. for example, melting point, electrical conductivity, appearance at room. If you see signs of a chemical reaction, the characteristic you are measuring is most likely a chemical property. if these signs are absent, the characteristic is probably a physical property. learn how to distinguish between a chemical property and a physical property of matter. here's the explanation of the distinction, with examples. Physical changes. a physical change is a change in matter that alters its form but not its chemical identity. the size or shape of matter often changes, but there is no chemical reaction. phase changes are physical changes. these include melting, boiling, vaporization, freezing, sublimation and deposition. breaking, crumpling, or molding matter.

Comments are closed.