Eyenuk Inc Receives Health Canada Approval For Eyeartв ў An Ai E

Eyenuk Inc Receives Health Canada Approval For Eyeartв ў An A Eyenuk, inc., headquartered in los angeles, california, is an ai diagnostic company focused on quickly and accurately identifying patients suffering from potentially blinding eye diseases and. Eyenuk, inc. receives health canada approval for eyeart™, an ai enabled cloud based automated diabetic retinopathy screening software march 8, 2018 march 8, 2018.

Eyenuk Receives Health Canada Approval For Eyeart Immediate, fully automated, on site diabetic retinopathy screening report in 60 seconds. no human grading needed. watch video experience eyeart eyeart® ai eye screening system eyeart is the first and only fda cleared ai technology for autonomous. Los angeles, june 22, 2023 – eyenuk, a global artificial intelligence (ai) digital health company, and the leader in real world applications for ai eye screening™ and ai predictive biomarkers™, has received u.s. food and drug administration (fda) clearance to use the topcon nw400 retinal camera with its eyeart ai system to automatically. Eyeart is already approved in canada and europe, and is the second ai based dr screening platform to receive the fda’s green light after the idx dr system in april 2018. in the united states, the platform is designed for use with 2 non mydriatic fundus cameras: canon cr 2 af and canon cr 2 plus af. Eyenuk inc.’s first product, the eyeart ai eye screening system, is an artificial intelligence (ai) technology for autonomous detection of diabetic retinopathy that’s been tested in more than 500,000 patient visits globally with more than 2 million images collected in real world clinical environments.
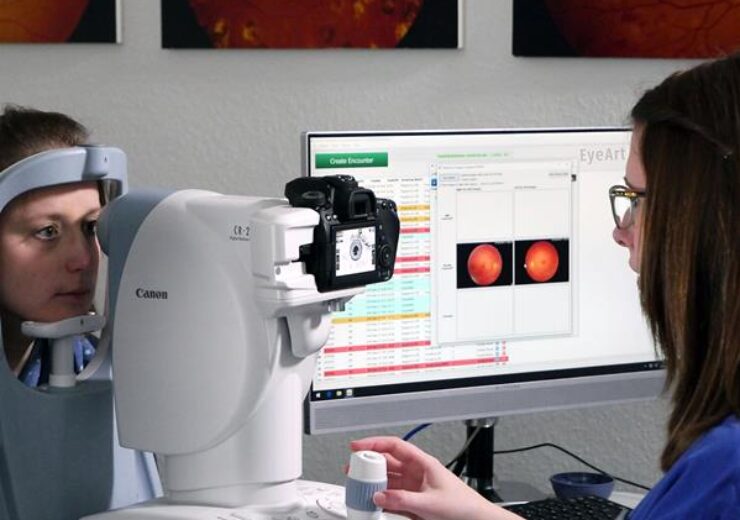
юааeyenukюабтащs юааeyeartюаб юааaiюаб System Offers Superior Detection Of Dr Study Says Eyeart is already approved in canada and europe, and is the second ai based dr screening platform to receive the fda’s green light after the idx dr system in april 2018. in the united states, the platform is designed for use with 2 non mydriatic fundus cameras: canon cr 2 af and canon cr 2 plus af. Eyenuk inc.’s first product, the eyeart ai eye screening system, is an artificial intelligence (ai) technology for autonomous detection of diabetic retinopathy that’s been tested in more than 500,000 patient visits globally with more than 2 million images collected in real world clinical environments. Eyenuk received fda clearance to use an additional retinal camera for the detection of diabetic retinopathy (dr) that is easier to use and make it easier for primary care clinics to adopt the technology. according to a news release, the company can now use the topcon nw400 retinal camera with its eyeart ai system to automatically detect. About eyenuk, inc. eyenuk, inc. is a global artificial intelligence (ai) digital health company and the leader in real world ai eye screening™ for autonomous disease detection and ai predictive.

Comments are closed.