Fda Grants Priority Review To Pfizer Biontech Covid 19 Vaccine
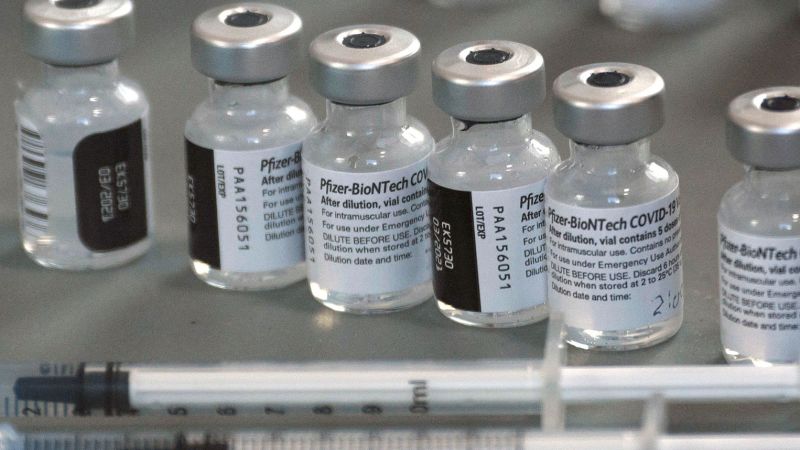
Fda Grants Priority Review To Pfizer Biontech Covid 19 Vaccine New york and mainz, germany, july 16, 2021—pfizer inc. (nyse: pfe) and biontech se (nasdaq: bntx) today announced that the u.s. food and drug administration (fda) granted priority review designation for the biologics license application (bla) for their mrna vaccine to prevent covid 19 in individuals 16 years of age and older. Pfizer and biontech said friday the fda has granted priority review designation to their application for approval of their covid 19 vaccine, and an fda official said the decision will come within.

Pfizer Biontech Covid 19 Vaccine Recommended For Cdc Immunization Sche Individuals 6 months through 4 years of age who have previously been vaccinated against covid 19 are eligible to receive one or two doses of the updated, authorized moderna or pfizer biontech. The pfizer biontech covid 19 vaccine (2023 2024 formula) is authorized for all doses administered to individuals 6 months through 11 years of age to prevent covid 19. Fda grants priority review for pfizer, biontech covid vaccine currently, pfizer and biontech's covid 19 vaccine has emergency use authorization (eau) in the united states for individuals 12 and older. Fda office of media affairs. 301 796 4540. consumer: 888 info fda. fda approved the first covid 19 vaccine, which has been known as the pfizer biontech covid 19 vaccine, and is now marketed as.

Comments are closed.