Fractional Distillation Labeled Diagram

Fractional Distillation Vector Illustration Labeled Educational Stock Relating what happens in the fractionating column to the phase diagram. suppose you boil a mixture with composition c 1. the vapor over the top of the boiling liquid will be richer in the more volatile component, and will have the composition c 2. that vapor now starts to travel up the fractionating column. Fractional distillation is a type of distillation which involves the separation of miscible liquids. the process involves repeated distillations and condensations and the mixture is usually separated into component parts. the separation happens when the mixture is heated at a certain temperature where fractions of the mixture start to vaporize.
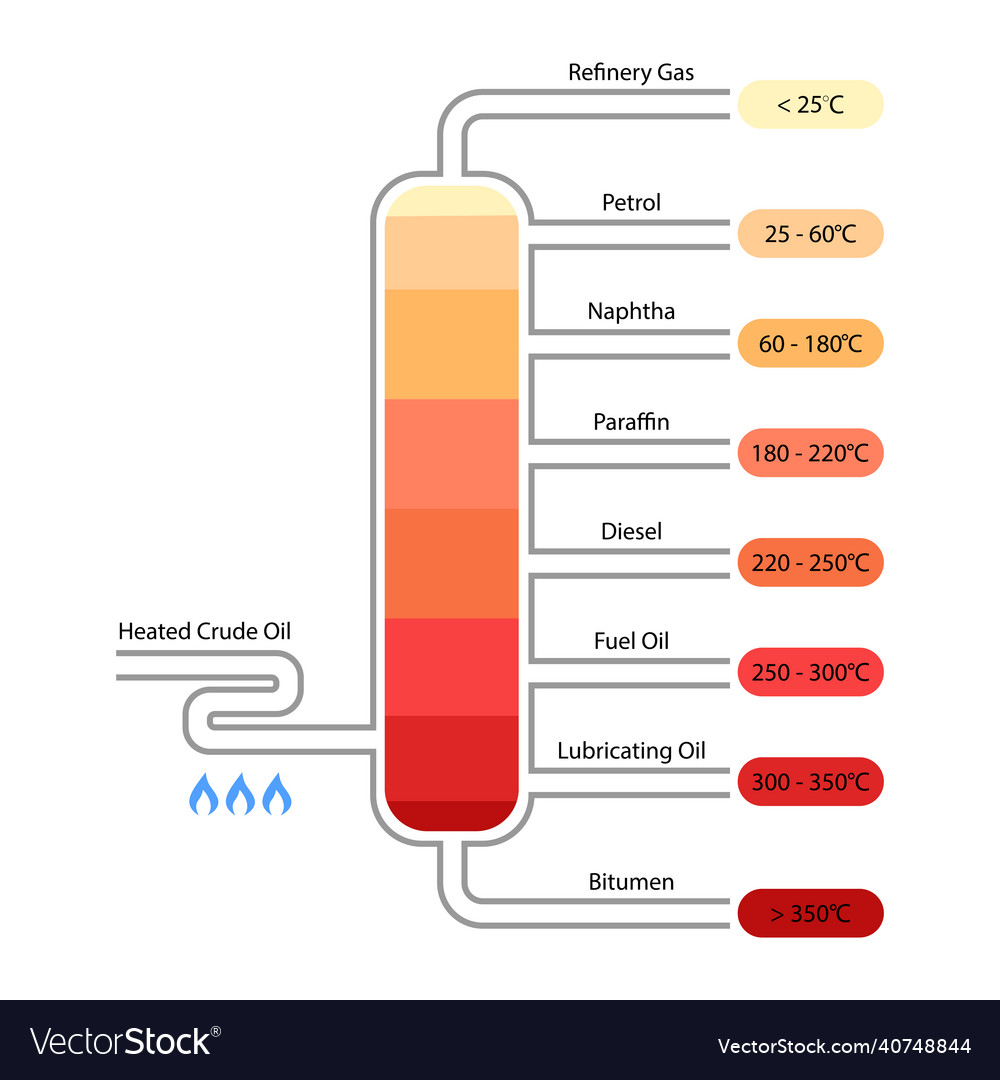
Crude Oil Fractional Distillation Labeled Diagram Vector Image Figure 1. diagram of a fractional distillation tower, showing where the different fractions will condense. note that the temperature is higher at the bottom, so the longer carbon chains will fall out at the bottom, the shorter carbon chains will go up the column until they hit a temperature at which they become liquid. Droplets of liquid should be seen in the fractional column, but there should never be a large pool of liquid (flooding). if the column floods, allow the liquid to drain back into the distilling flask and heat at a gentler rate. this page titled 5.3d: step by step procedures for fractional distillation is shared under a cc by nc nd 4.0 license. A typical lab fractional distillation would look like this: some notes on the apparatus. the fractionating column is packed with glass beads (or something similar) to give the maximum possible surface area for vapour to condense on. you will see why this is important in a minute. some fractionating columns have spikes of glass sticking out from. 5.3c: uses of fractional distillation. fractional distillation is used for both oil refining and purification of reagents and products. fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and therefore similar molecular weights and properties.

Draw A Well Labelled Diagram Showing The Process Of Fractional A typical lab fractional distillation would look like this: some notes on the apparatus. the fractionating column is packed with glass beads (or something similar) to give the maximum possible surface area for vapour to condense on. you will see why this is important in a minute. some fractionating columns have spikes of glass sticking out from. 5.3c: uses of fractional distillation. fractional distillation is used for both oil refining and purification of reagents and products. fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and therefore similar molecular weights and properties. Fractional distillation in a laboratory makes use of common laboratory glassware and apparatuses, typically including a bunsen burner, a round bottomed flask and a condenser, as well as the single purpose fractionating column. fractional distillation. as an example, consider the distillation of a mixture of water and ethanol. ethanol boils at. Microsoft word 2219 exp6 fractional distillation procedure ws23.docx. objective: in this experiment you will learn to separate the components of a solvent mixture (two liquids) by distillation. the boiling point (bp) range and refractive index (ri) will be determined for the three fractions. nmr will be used to determine the percent.
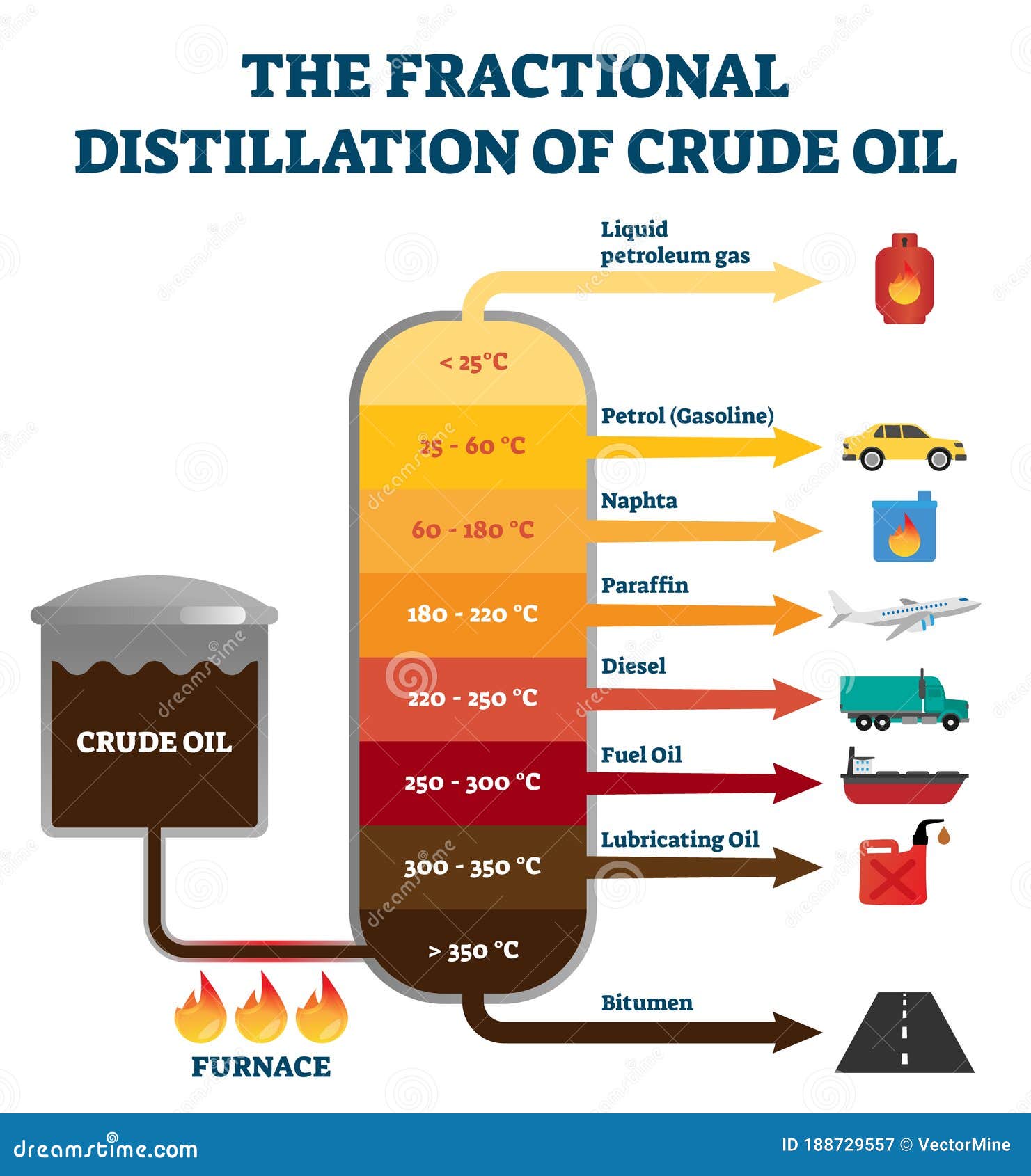
Fractional Distillation Of Crude Oil Labeled Educational Explanation Fractional distillation in a laboratory makes use of common laboratory glassware and apparatuses, typically including a bunsen burner, a round bottomed flask and a condenser, as well as the single purpose fractionating column. fractional distillation. as an example, consider the distillation of a mixture of water and ethanol. ethanol boils at. Microsoft word 2219 exp6 fractional distillation procedure ws23.docx. objective: in this experiment you will learn to separate the components of a solvent mixture (two liquids) by distillation. the boiling point (bp) range and refractive index (ri) will be determined for the three fractions. nmr will be used to determine the percent.

Comments are closed.