Gmp Process Validation Protocol At Jeanne Mcelwee Blog
Gmp Process Validation Protocol At Jeanne Mcelwee Blog Process validation: general principles and practices. additional copies are available from: office of communications division of drug information, wo51, room 2201 10903 new hampshire ave. silver. 1 2 october 2024. process validation live online training. register now for eca's gmp newsletter. "bracketing" during process validation has become common practice. in the usa, bracketing has been practiced for some time, and the topic was included in the annex 15 revision in 2015 (chapter 5.4.). according to annex 15, it is a science and.
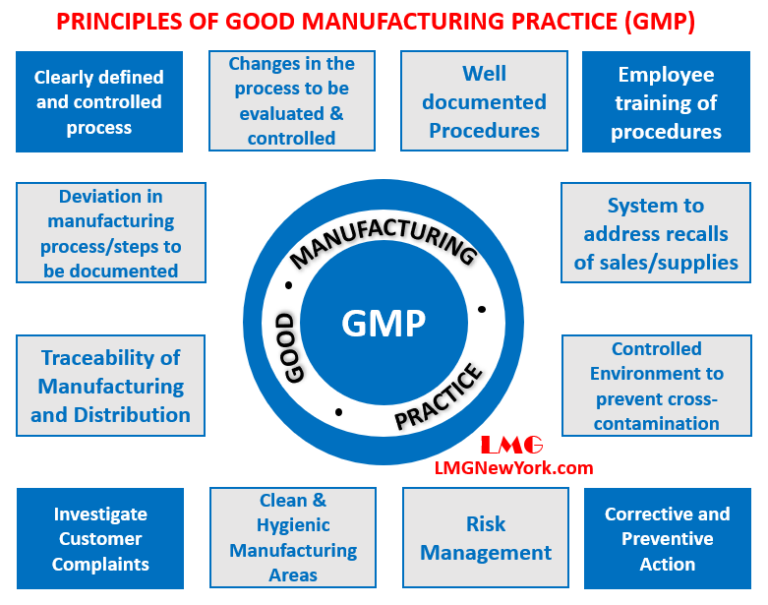
Gmp Process Validation Protocol At Jeanne Mcelwee Blog This document comprises individual recommendations on four topics relating to equipment qualification and process validation in pharmaceutical manufacture, as follows: validation master plan installation and operational qualification non sterile process validation cleaning validation the four recommendations comprising …. Gmp process validation protocol at jeanne mcelwee blog. gmp process validation protocol. web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. web the overarching text presented in this annex constitutes the general principles of the new guidance on. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence through a series of processes to confirm that a particular manufacturing process will consistently produce products that meet predefined quality standards. gmp validation plays a crucial role in ensuring that pharmaceutical. 4.1 qa personnel – to allot protocol report number and to prepare the validation protocol report. 4.2 validation team (consisting of head or their designee of production, quality control, quality assurance, – to review, approve the protocol and certify the final reports. 4.3 production, qc and qa personnel – execution of the process.
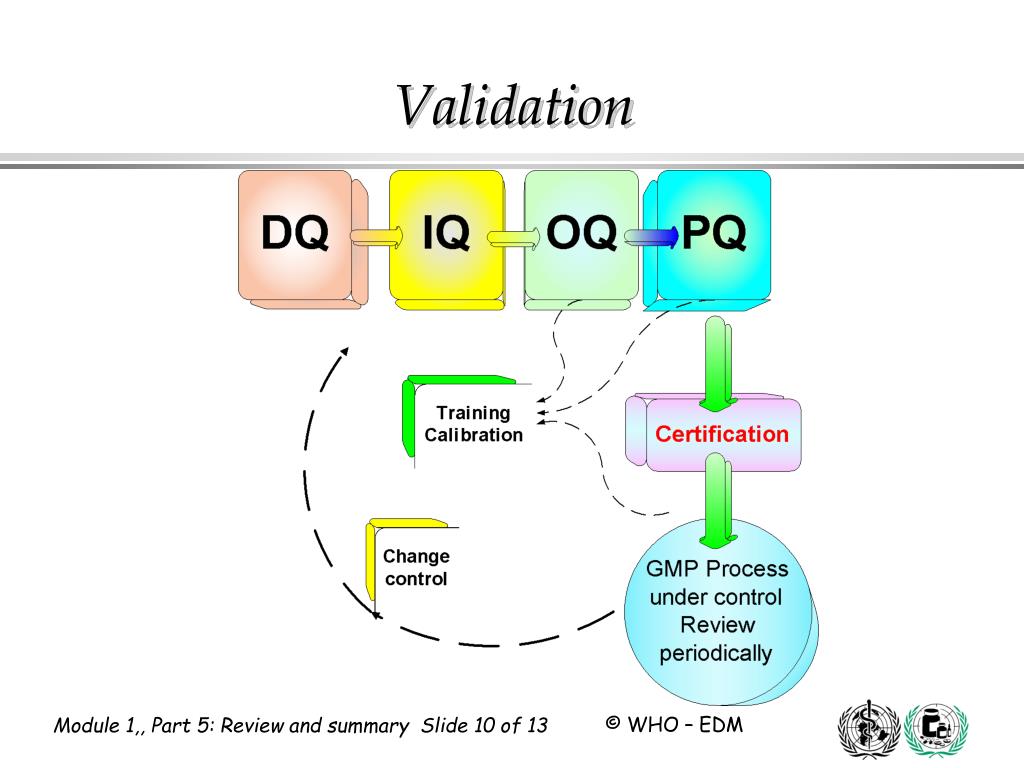
Gmp Process Validation Protocol At Jeanne Mcelwee Blog Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence through a series of processes to confirm that a particular manufacturing process will consistently produce products that meet predefined quality standards. gmp validation plays a crucial role in ensuring that pharmaceutical. 4.1 qa personnel – to allot protocol report number and to prepare the validation protocol report. 4.2 validation team (consisting of head or their designee of production, quality control, quality assurance, – to review, approve the protocol and certify the final reports. 4.3 production, qc and qa personnel – execution of the process. Gmp requirements for process validation continue throughout the lifecycle of the process 5.2.2 this approach should be applied to link product and process development. it will ensure validation of the commercial manufacturing process and maintenance of the process in a state of control during routine commercial production. This process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical services department for the performance of this protocol. detail if prospective, concurrent or retrospective approach and describe the release for sale mechanism. detail the number of batches to be.
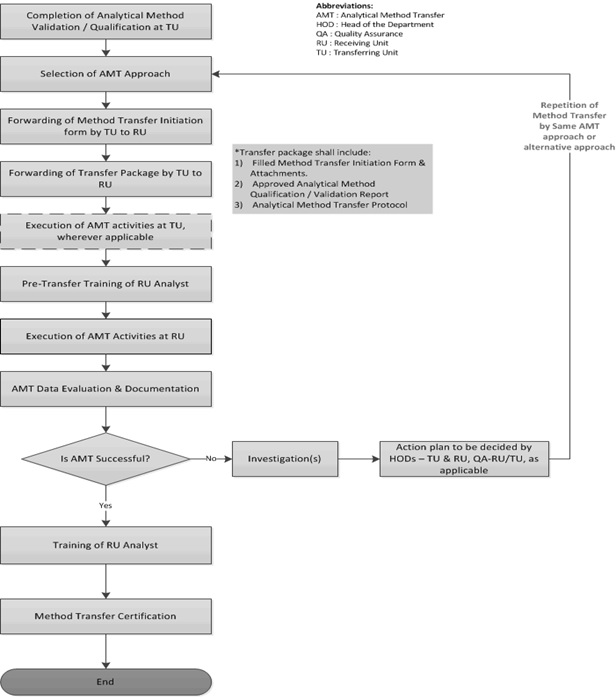
Gmp Process Validation Protocol At Jeanne Mcelwee Blog Gmp requirements for process validation continue throughout the lifecycle of the process 5.2.2 this approach should be applied to link product and process development. it will ensure validation of the commercial manufacturing process and maintenance of the process in a state of control during routine commercial production. This process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical services department for the performance of this protocol. detail if prospective, concurrent or retrospective approach and describe the release for sale mechanism. detail the number of batches to be.

Gmp Process Validation Protocol At Jeanne Mcelwee Blog

Comments are closed.