Good Manufacturing Practices Gmp
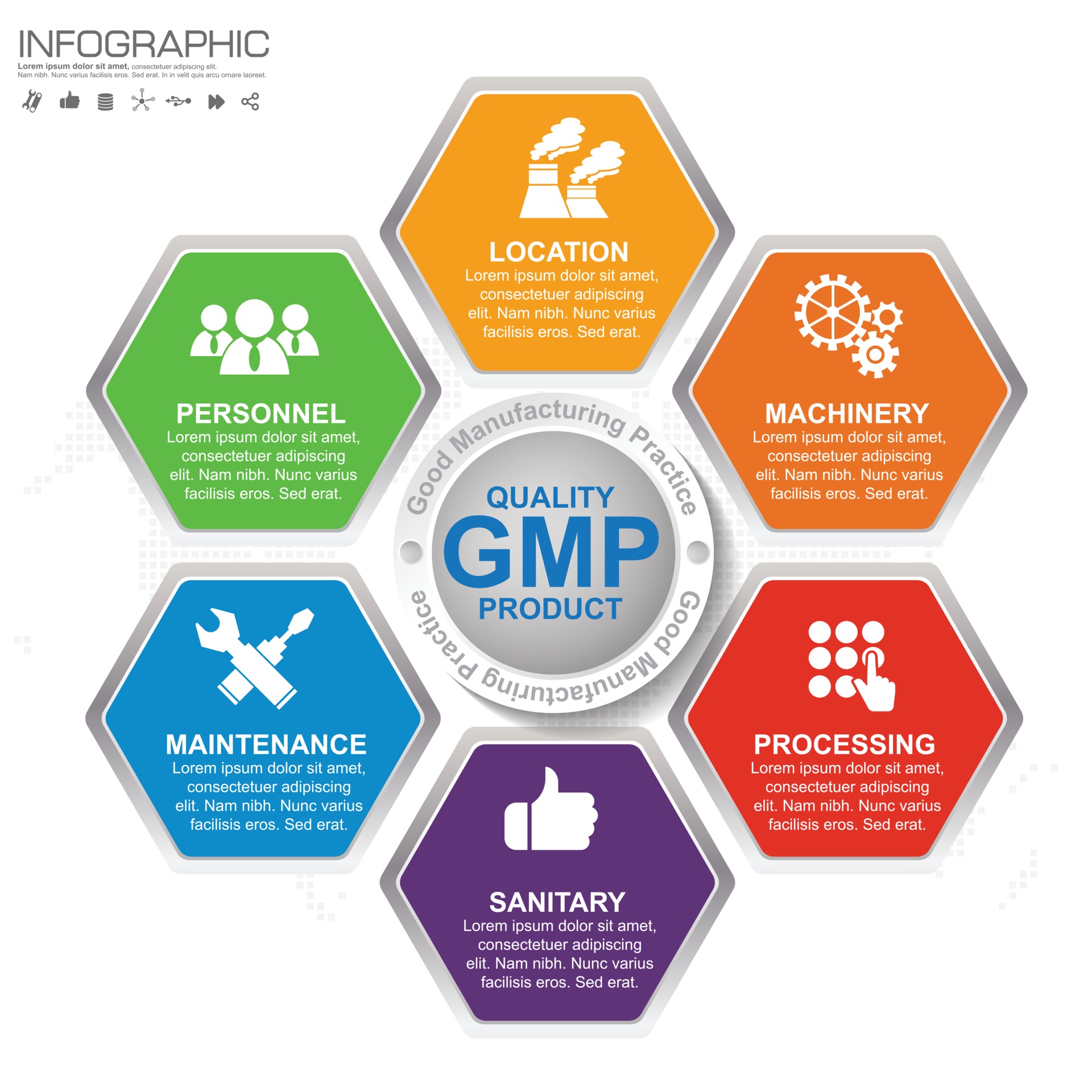
Gmp Good Manufacturing Practice 6 Heading Of Infographic Template With Gmp, which stands for good manufacturing practices, is a system that ensures that manufactured products—such as food, cosmetics, and pharmaceutical goods—are consistently produced and controlled according to set quality standards. implementing gmp can help cut down on losses and waste, and avoid recalls, fines, and jail time. Learn how fda ensures the quality and safety of drug products by monitoring drug manufacturers' compliance with cgmp regulations. find links to cgmp regulations, guidance documents, and resources for different product types and manufacturing considerations.
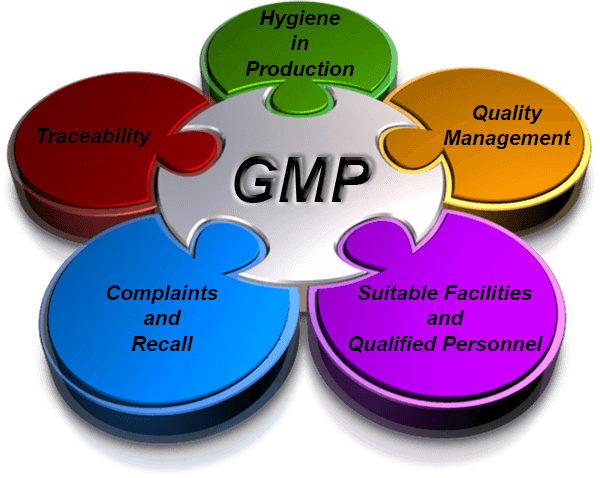
Good Manufacturing Practice Regulations Changes Ispe provides comprehensive resources on good manufacturing practice (gmp), a system for ensuring quality standards in pharmaceutical production. find gmp regulations, guidelines, audits, and community of practice by topic and country. Cgmp refers to the current good manufacturing practice regulations enforced by the fda. cgmp provides for systems that assure proper design, monitoring, and control of manufacturing processes and. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance that ensures that medicinal products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the product specification. Learn about the eu standards and requirements for medicines manufacturing and distribution. find guidance, legal framework, inspections and cooperation on gmp and gdp.
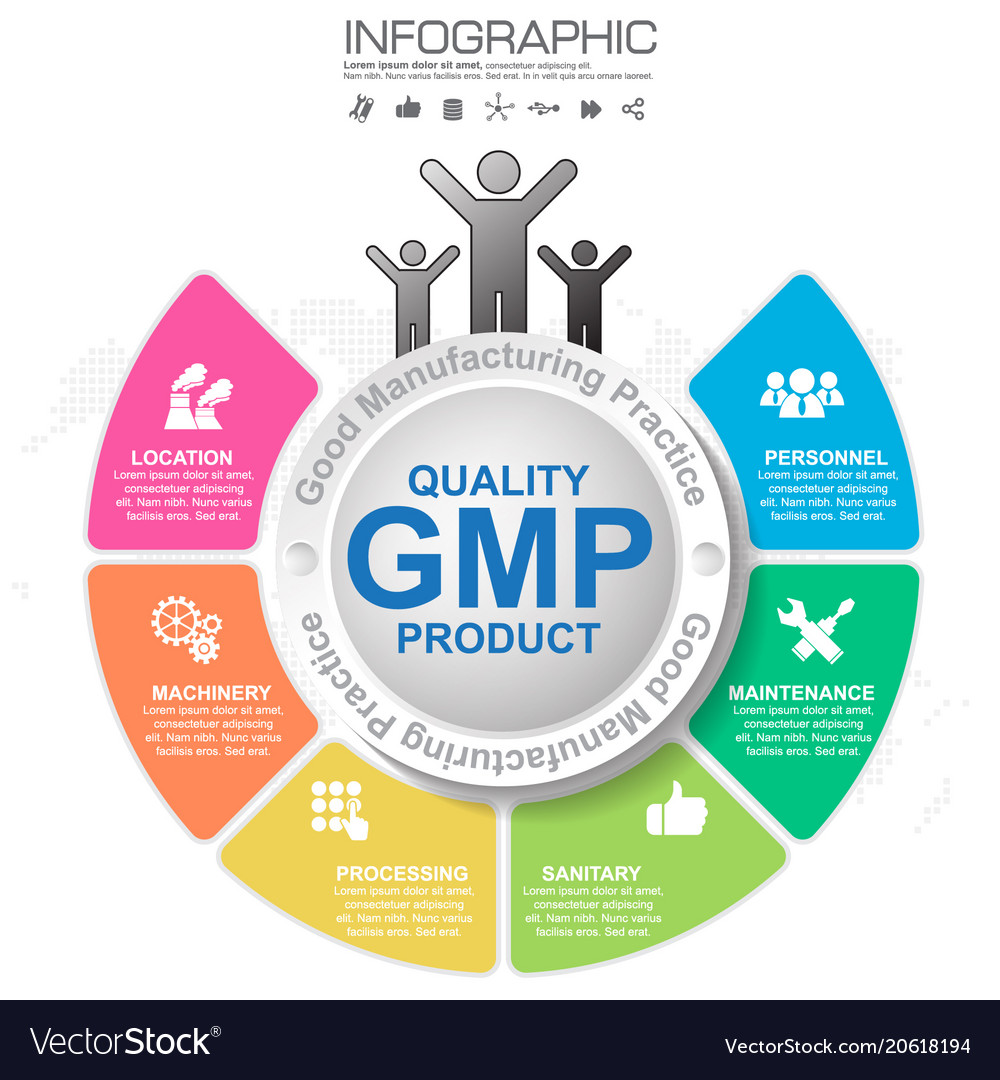
Gmp Good Manufacturing Practice 6 Heading Of Vector Image Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance that ensures that medicinal products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the product specification. Learn about the eu standards and requirements for medicines manufacturing and distribution. find guidance, legal framework, inspections and cooperation on gmp and gdp. Gmp stands for good manufacturing practice, a set of regulations that ensure the safety, purity, and effectiveness of drugs, medical devices, some food, and blood. ispe provides education, training, and resources to help professionals and organizations comply with gmp. Following current good manufacturing practices (cgmps) help to ensure the safety of food. cgmp regulations generally address matters including appropriate personal hygienic practices, design and.

Comments are closed.