Group Separation Part 3a Qualitative Analysis Of Group 3

Group Separation Part 3b Qualitative Analysis Of Group 3 Y Separation of al3 from cr3 . slowly add 6 m acetic acid to the supernatant solution saved from step 5 until it is acidic (test with blue litmus paper). add 6 m nh 3 one drop at a time until the solution just becomes basic again (test with red litmus paper). now add twenty additional drops of excess nh 3. To download the slides click on the link given below: drive.google file d 1xtrcjubvkzwg4ux7d5gb9wohrnumeuv3 view?usp=sharingin this video i am dis.

Solved Analysis Of Group Iii Cations Qualitative Analysis Is Chegg Filter this precipitate and dissolve a small portion of it into 1ml hot dil hcl. cool and add 1ml 6m ammonium acetate solution and 0.5ml of 1m aluminon reagent (0.1g tri ammonium arurine tricarboxylate o(coonh 4)c 6 h 3 =c[c 6 h 3 (oh) coonh 4] 2 dissolved in 100ml water), stir it and add ammonium carbonate solution. a red coloured precipitate confirms the presence of al(iii). E same time for the presence of group iii cations and use about 20 24 drops in your analysis.step 2: oxidation of cr(iii) to cr(vi) an. separation of insoluble hydroxides: add 1 ml. of 6 m naoh to the solution in a 30 ml beaker. boil v. ry gently for 1 minute while stirring. remove heat and slo. y add dropwise 1 ml of 1 m nac. Figure 5.1.1: precipitation of iron ions and chromium ions, as fe(oh) 3, fe(oh) 2, and cr(oh) 3 in the presence by oh − at ph ~9. the concentration of fe2 , i.e., the most soluble hydroxide of group iii cations, is reduced by more than 99.99%, i.e., from 0.1m to 4.9 x 10 7 m when ph is increased to 9 and oh − concentration is increased to. This page titled 1.5: separation of cations in groups is shared under a public domain license and was authored, remixed, and or curated by muhammad arif malik. cations commonly found in water are separated into five groups by adding suitable reagents that selectively precipitate a set of cations. group i is separated as insoluble chlorides.

Qualitative Analysis Docx Qualitative Analysis Of Group I Cations E7 Figure 5.1.1: precipitation of iron ions and chromium ions, as fe(oh) 3, fe(oh) 2, and cr(oh) 3 in the presence by oh − at ph ~9. the concentration of fe2 , i.e., the most soluble hydroxide of group iii cations, is reduced by more than 99.99%, i.e., from 0.1m to 4.9 x 10 7 m when ph is increased to 9 and oh − concentration is increased to. This page titled 1.5: separation of cations in groups is shared under a public domain license and was authored, remixed, and or curated by muhammad arif malik. cations commonly found in water are separated into five groups by adding suitable reagents that selectively precipitate a set of cations. group i is separated as insoluble chlorides. Also complete an analysis summary in your notebook similar to table 3 (a form for this summary is provided in part b of the report form). directions for steps 1 5 were given in experiment 7, and are related to group i cation analysis only. 6. precipitation of fe(oh) 3 and al(oh) 3 to 1 ml of the solution to be tested, add an excess (about. Cation group separation introduction. qualitative analysis is a procedure for identifying substances in a mixture. in this experiment you will be identifying cations present in a solution. these ions are identified by specific chemical tests but because one cation can interfere with a test for another ion, the ions must first be separated.
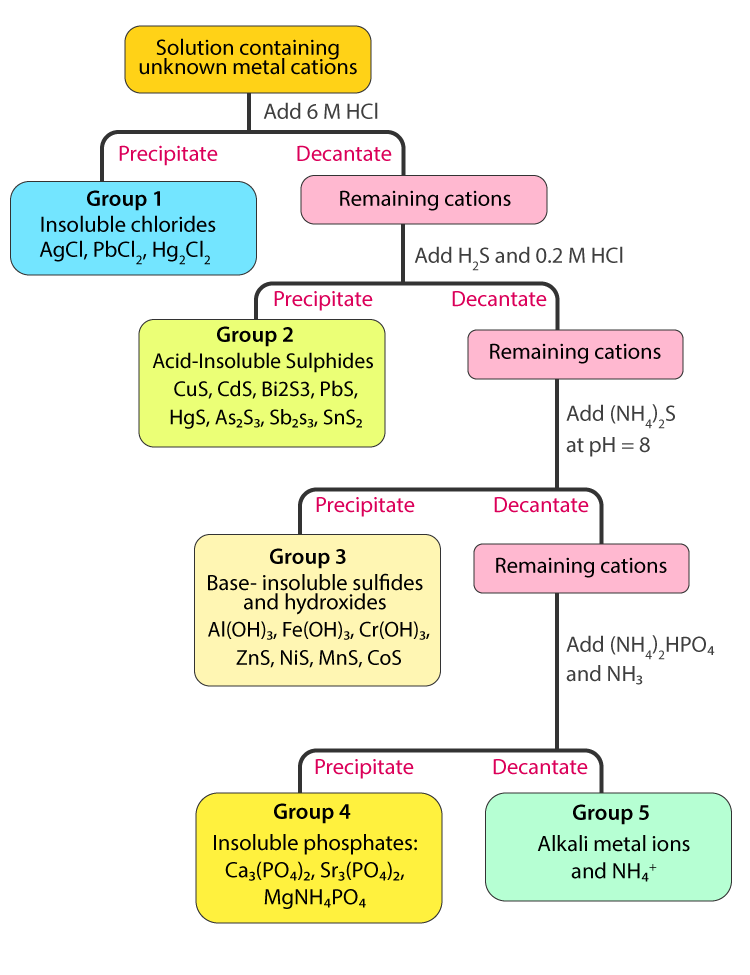
Qualitative Analysis Cations Flow Chart Also complete an analysis summary in your notebook similar to table 3 (a form for this summary is provided in part b of the report form). directions for steps 1 5 were given in experiment 7, and are related to group i cation analysis only. 6. precipitation of fe(oh) 3 and al(oh) 3 to 1 ml of the solution to be tested, add an excess (about. Cation group separation introduction. qualitative analysis is a procedure for identifying substances in a mixture. in this experiment you will be identifying cations present in a solution. these ions are identified by specific chemical tests but because one cation can interfere with a test for another ion, the ions must first be separated.
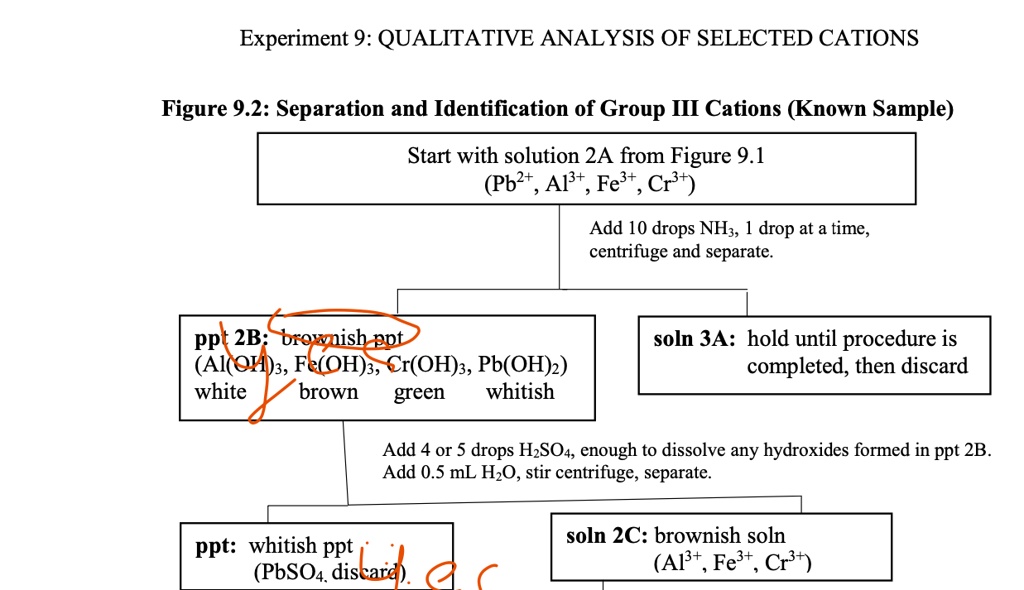
Solved Experiment 9 Qualitative Analysis Of Selected Cations Figure 9

Comments are closed.