Heating And Cooling Curve Explanation
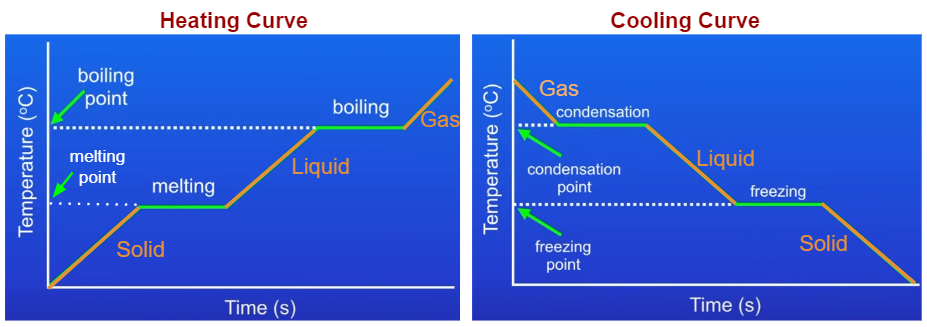
Heating And Cooling Graphs Examples Solutions Videos Notes The cooling curve and the heating curve are essentially the same curve but viewed in reverse. when studying a heating curve, we observe how a substance changes from solid to liquid, eventually to. The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant.

Heating And Cooling Curves Explained The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2 . the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would. Heating and cooling curves. the experimental set up we imagined would generate a heating curve. heating and cooling curves are graphs. they plot a substance's temperature (y axis) against heat (x axis). for heating curves, we start with a solid and add heat energy. for cooling curves, we start with the gas phase and remove heat energy. Considering the definition of boiling point, plots of vapor pressure versus temperature represent how the boiling point of the liquid varies with pressure. also described was the use of heating and cooling curves to determine a substance’s melting (or freezing) point. 11.12 : heating and cooling curves. when a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and phase of the substance is observed; this is graphically represented by heating and cooling curves. for instance, the addition of heat raises the temperature of a solid; the amount of heat.

Heating And Cooling Curve Introduction Plus Kinetic And Potential Considering the definition of boiling point, plots of vapor pressure versus temperature represent how the boiling point of the liquid varies with pressure. also described was the use of heating and cooling curves to determine a substance’s melting (or freezing) point. 11.12 : heating and cooling curves. when a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and phase of the substance is observed; this is graphically represented by heating and cooling curves. for instance, the addition of heat raises the temperature of a solid; the amount of heat. Ethyl chloride (c2h5cl) boils at 12 °c. when liquid c2h5cl under pressure is sprayed on a room temperature (25 °c) surface in air, the surface is cooled considerably. (b) assume that the heat lost by the surface is gained by ethyl chloride. Short summary. a heating curve is a graphical representation of the relationship between the temperature and heat of a substance. it can be broken down into five stages:the temperature at which a.

Heating And Cooling Curves вђ Overview Examples Expii Ethyl chloride (c2h5cl) boils at 12 °c. when liquid c2h5cl under pressure is sprayed on a room temperature (25 °c) surface in air, the surface is cooled considerably. (b) assume that the heat lost by the surface is gained by ethyl chloride. Short summary. a heating curve is a graphical representation of the relationship between the temperature and heat of a substance. it can be broken down into five stages:the temperature at which a.

Heating And Cooling Curve Explanation

Comments are closed.