Heating Curves And Cooling Curves

Heating And Cooling Curves Explained The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2 . the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would.
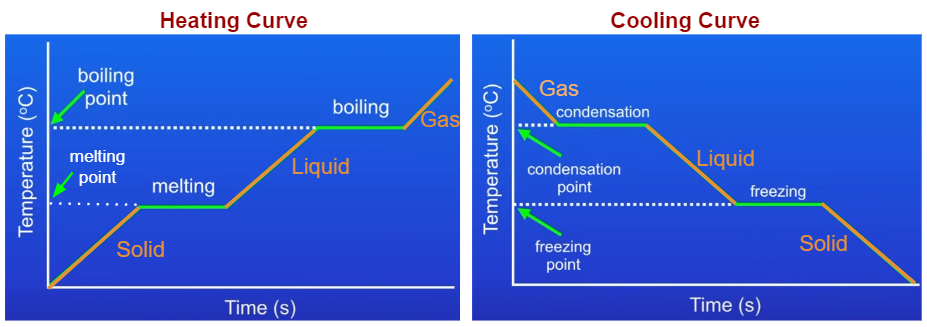
Heating And Cooling Graphs Examples Solutions Videos Notes Figure \(\pageindex{1}\): a typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. plateaus in the curve (regions of constant temperature) are exhibited when the substance undergoes phase transitions. consider the example of heating a pot of water to boiling. In the science world, we use heating and cooling curves to model such physical changes. a heating or cooling curve is a simple line graph that shows the phase changes a given substance undergoes. The cooling curve, a plot of temperature versus cooling time, in figure \(\pageindex{4}\) plots temperature versus time as a 75 g sample of steam, initially at 1 atm and 200°c, is cooled. although we might expect the cooling curve to be the mirror image of the heating curve in figure \(\pageindex{3}\), the cooling curve is not an identical. Heating cooling curves. a typical heating curve consists of a horizontal axis representing time and a vertical axis representing temperature. the curve is divided into distinct segments, each corresponding to a specific phase of the substance. during heating, the substance undergoes different phase transitions, such as solid to liquid (melting.

Heating And Cooling Curves вђ Overview Examples Expii The cooling curve, a plot of temperature versus cooling time, in figure \(\pageindex{4}\) plots temperature versus time as a 75 g sample of steam, initially at 1 atm and 200°c, is cooled. although we might expect the cooling curve to be the mirror image of the heating curve in figure \(\pageindex{3}\), the cooling curve is not an identical. Heating cooling curves. a typical heating curve consists of a horizontal axis representing time and a vertical axis representing temperature. the curve is divided into distinct segments, each corresponding to a specific phase of the substance. during heating, the substance undergoes different phase transitions, such as solid to liquid (melting. 11.12 : heating and cooling curves. when a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and phase of the substance is observed; this is graphically represented by heating and cooling curves. for instance, the addition of heat raises the temperature of a solid; the amount of heat. Understanding heating and cooling curves is crucial for grasping how substances absorb or release heat during phase changes. as a substance heats up, it undergoes an endothermic process, indicated by a positive heat variable (q), absorbing energy to break molecular bonds and transition from solid to liquid (melting or fusion) and eventually to gas (vaporization).

Heating And Cooling Curve Introduction Plus Kinetic And Potential 11.12 : heating and cooling curves. when a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and phase of the substance is observed; this is graphically represented by heating and cooling curves. for instance, the addition of heat raises the temperature of a solid; the amount of heat. Understanding heating and cooling curves is crucial for grasping how substances absorb or release heat during phase changes. as a substance heats up, it undergoes an endothermic process, indicated by a positive heat variable (q), absorbing energy to break molecular bonds and transition from solid to liquid (melting or fusion) and eventually to gas (vaporization).

Comments are closed.