How Does Simple Distillation Work The Engineer S Perspective
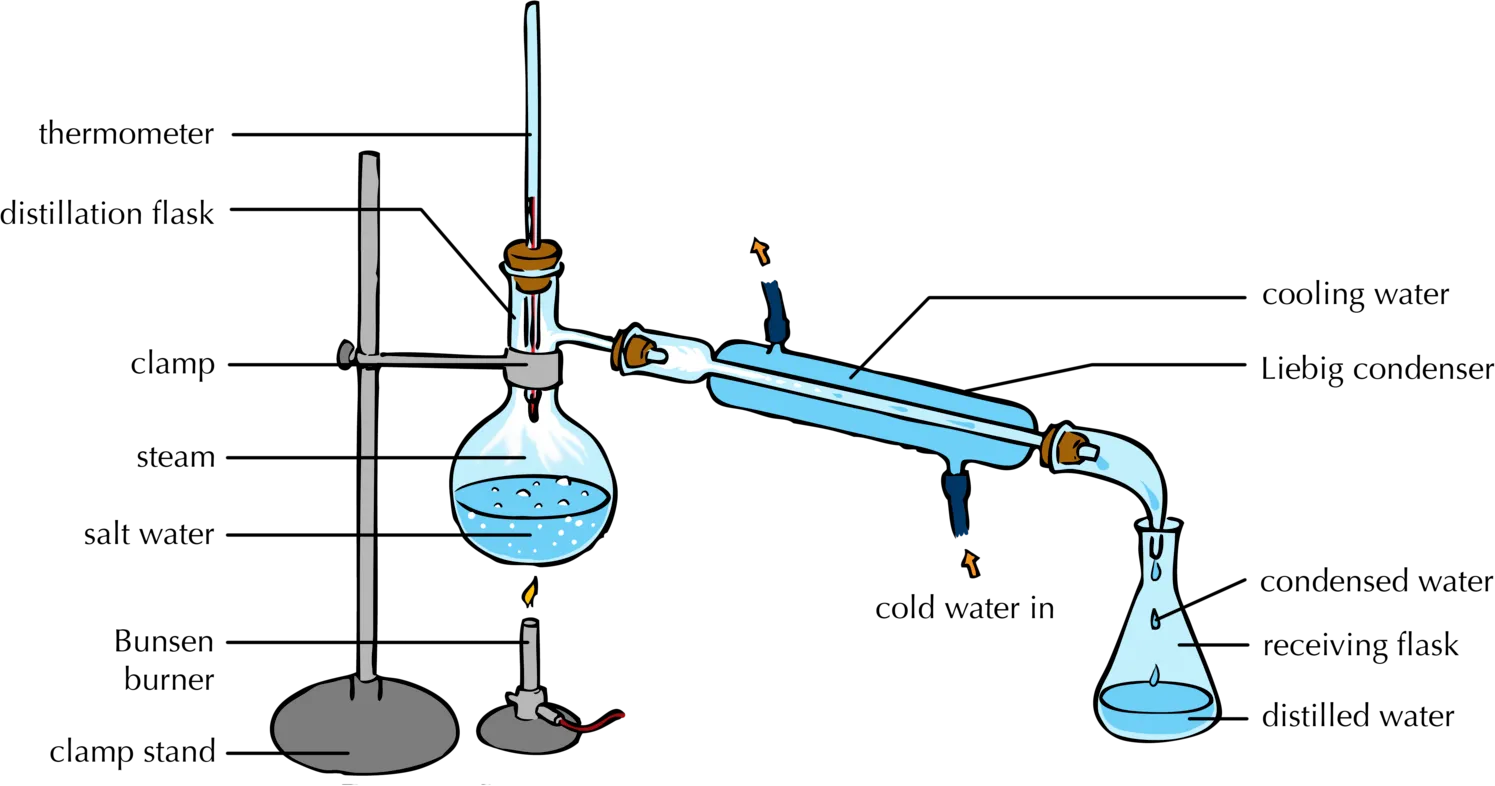
How Does Simple Distillation Work The Engineer S Perspective Simple distillation will be effective in this case because pure water (solvent) vaporizes at 100°c under normal conditions while the salt (solute) is non volatile. the saltwater in the distillation flask is heated until the mixture boils and water evaporates as steam. as steam rises, it enters the attached condenser where cooling water passes. The main difference between simple and fractional distillation is the former is best used when the boiling points of two liquids are significantly different from each other, or to separate liquids from non volatile solid components. meanwhile, fractional distillation is best used when the boiling points of two or more liquid components are.

What Is The Distillation Process The Chemistry Blog Distillation is a separation process based on differences in boiling temperatures or relative volatility of liquid liquid solutions. in a simple distillation set up, the starting feed mixture has one more volatile component than the other. due to large differences in boiling points (at least 100॰c), it is easier to separate the vapor rich. Attach hoses to the condenser for water cooling, making sure that they are secure. remember, water goes in at the bottom and out at the top. open the water flow to a little more than a trickle. do not open the faucet to full flow, or you will risk a flood. 8. set the stirring speed knob to about mid range. Cryogenic distillation (also known as low temperature rectification) is a separation process that works by liquifying the gas mixture at very low temperatures and then selectively distilling the specific gas component at its boiling point. though the process yields products of high purity, it is energy intensive due to purification requirements. Distillation is a widely used method for separating mixtures based on differences in the conditions required to change the phase of components of the mixture. to separate a mixture of liquids, the liquid can be heated to force components, which have different boiling points, into the gas phase. the gas is then condensed back into liquid form.

Comments are closed.