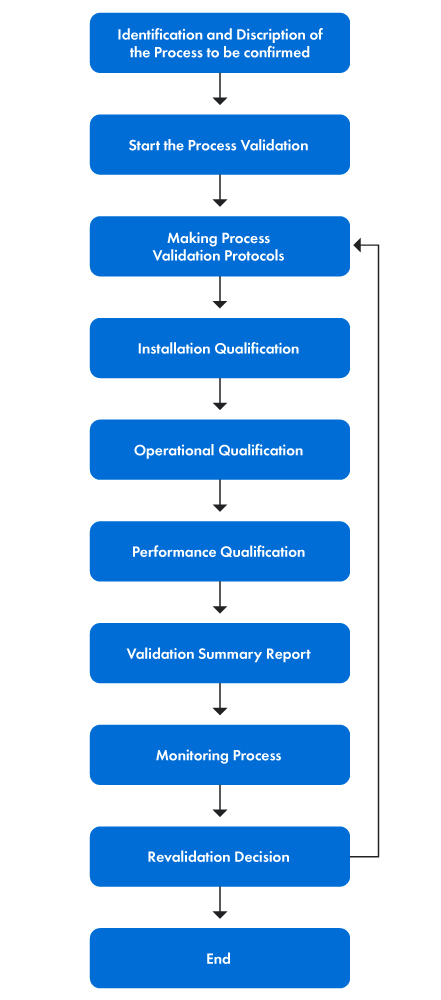
How To Write A Validation Protocol Different Parts Of Validation Protocol

How To Write A Validation Protocol Different Parts Of Validation A validation protocol is a crucial document that outlines the procedures and re in this video, we will learn step by step how to write a validation protocol. 4. reason for validation: the reason behind the validation due to which the validation of process or method is being done. if the product or method is new then the “new product” or “new method” should be written. 5. revalidation criteria: the situation in which we shall re validate the process should be mentioned. 6.
Writing Validation Protocols For Medical Devices Freyr Medical Devices Guidance for industry. 1. process validation: general principles and practices . this guidance represents the food and drug administration’s (fda’s) current thinking on this topic. Validation protocols can be hundreds of pages in length. templates themselves are typically around 50 to 60 pages long. elements of a validation protocol must include: product characteristics. a validation protocol must show what a system is meant to achieve or produce. manufacturing equipment. Validation for pharmaceutical process equipment and provides instructions on how to achieve it. with pragmatic approach, this book includes 38 useful protocol templates, already completed, that provide instant answers to most protocol writing and testing questions. Product and process development data are a prerequisite to securing a successful process validation. based on this data, a control philosophy is built to ensure that it achieves the desired product quality attributes. variations can severely affect product quality. so, it is important to: identify the variations.
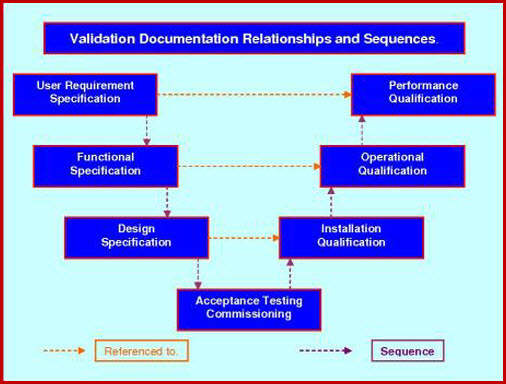
Validation Protocol Standards Fda Ec Who Pharma Medical Validation for pharmaceutical process equipment and provides instructions on how to achieve it. with pragmatic approach, this book includes 38 useful protocol templates, already completed, that provide instant answers to most protocol writing and testing questions. Product and process development data are a prerequisite to securing a successful process validation. based on this data, a control philosophy is built to ensure that it achieves the desired product quality attributes. variations can severely affect product quality. so, it is important to: identify the variations. The method validation experiments should be well planned and laid out to ensure efficient use of time and resources during execution of the method validation. the best way to ensure a well planned validation study is to write a method validation protocol that will be reviewed and signed by the appropriate person (e.g., laboratory management and. Validation protocol. a document describing the activities tobe performed during validation, including the acceptance criteria. validation report. a document in which the records, results and evaluation of validation are documented and summarized. it should also contain a conclusion of the outcome of the validation. ve. rification.

Strategies To Write Effective Validation Protocol Verification And The method validation experiments should be well planned and laid out to ensure efficient use of time and resources during execution of the method validation. the best way to ensure a well planned validation study is to write a method validation protocol that will be reviewed and signed by the appropriate person (e.g., laboratory management and. Validation protocol. a document describing the activities tobe performed during validation, including the acceptance criteria. validation report. a document in which the records, results and evaluation of validation are documented and summarized. it should also contain a conclusion of the outcome of the validation. ve. rification.
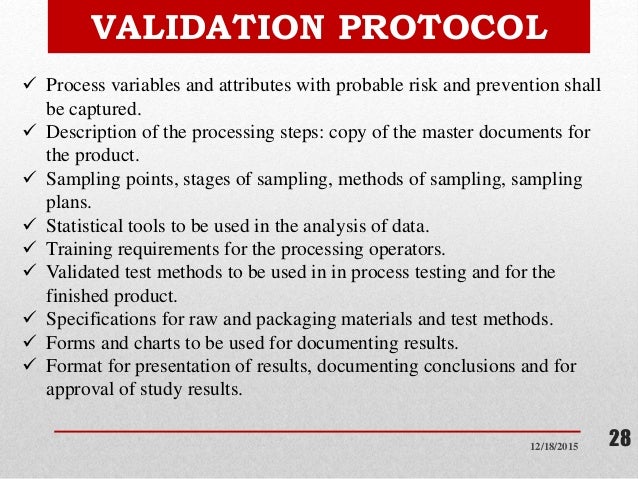
Process Validation

Comments are closed.