How To Write Ionic Formulas
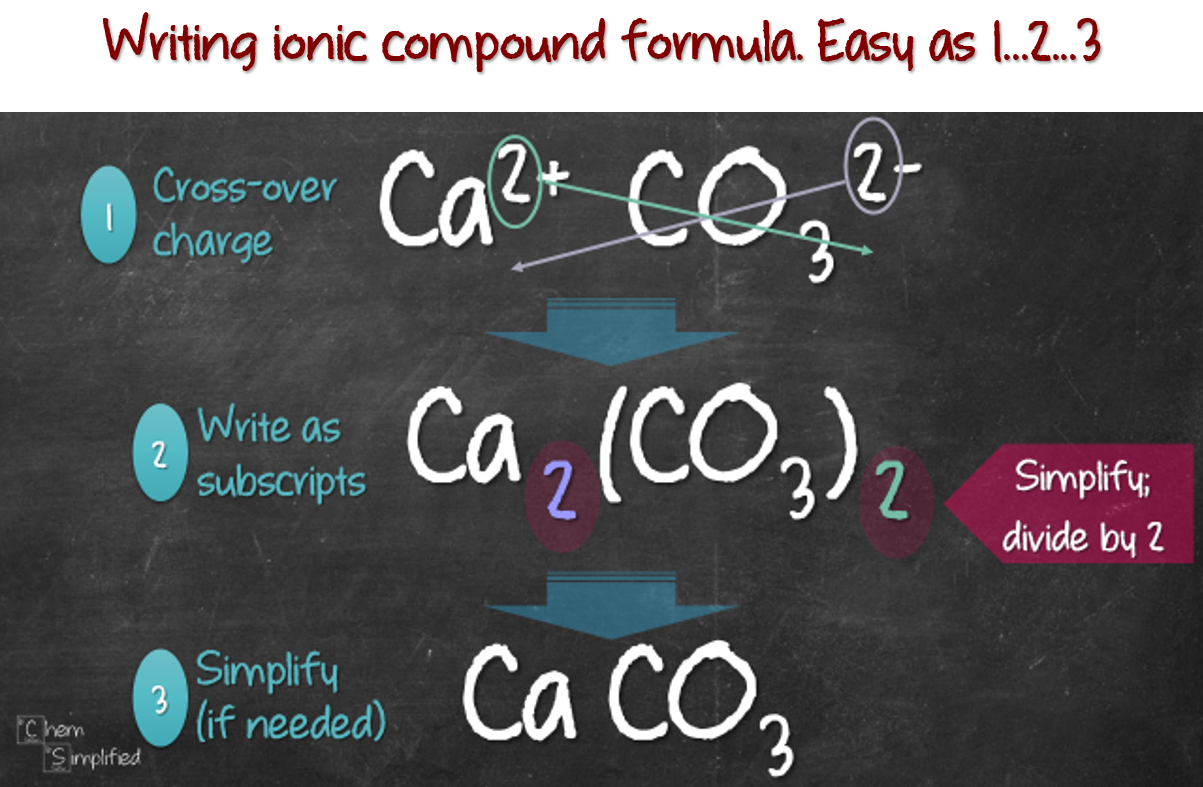
Writing Formula For Ionic Compounds вђ Chemsimplified Exercise 5.5.2 5.5. 2. write the chemical formula for an ionic compound composed of each pair of ions. the calcium ion and the oxygen ion. the 2 copper ion and the sulfur ion. the 1 copper ion and the sulfur ion. answer a: answer b: answer c: be aware that ionic compounds are empirical formulas and so must be written as the lowest ratio of. Learn how to write ionic formulas for binary and polyatomic compounds by balancing charges and using common multiples. see examples, questions and answers, and related topics on ionic bonds and formulas.

How To Write Formulas Of Ionic Compounds Write out the ions: cation on the left, anion on the right. cross the number in the charge over. write the crossed over number as subscripts. simplify if needed. it might be hard trying to imagine, so here’s how i apply this method on five examples. notice this method also works on polyatomic ions, just don’t forget to use bracket. This chemistry video tutorial explains how to write chemical formulas of ionic compounds including those with transition metals and polyatomic ions.chemistry. Mgso 4 · 7 h 2 o – magnesium sulfate hepta hydrate. notice that the number of water molecules is given by a prefix. for example, write the formula of calcium chloride hexahydrate. first, the formula of the salt itself: next, we add 6 water molecules according to the prefix “hexa”: cacl 2 · 6h 2 o. check also. When writing the ionic formula, we follow two additional conventions: write the formula for the cation first and the formula for the anion next, but. cross the charges down diagonally. thus, for the compound between na na and cl− cl −, we have the ionic formula nacl nacl (figure 4.6.1 4.6. 1).
.PNG)
Naming Ionic Compounds Mgso 4 · 7 h 2 o – magnesium sulfate hepta hydrate. notice that the number of water molecules is given by a prefix. for example, write the formula of calcium chloride hexahydrate. first, the formula of the salt itself: next, we add 6 water molecules according to the prefix “hexa”: cacl 2 · 6h 2 o. check also. When writing the ionic formula, we follow two additional conventions: write the formula for the cation first and the formula for the anion next, but. cross the charges down diagonally. thus, for the compound between na na and cl− cl −, we have the ionic formula nacl nacl (figure 4.6.1 4.6. 1). 1. identify a binary compound. the simplest type of ionic compound is made from exactly 2 elements, 1 metal and 1 non metal. their name is always written as 2 element names, plus the ide suffix attached to the second name. [1] examples of simple binary ionic compounds include potassium oxide and sodium phosphide. This chemistry video tutorial provides an introduction to writing the formula of an ionic compound that contains transition metals with roman numerals and po.

Naming And Writing Formulas For Ionic Compounds Youtube 1. identify a binary compound. the simplest type of ionic compound is made from exactly 2 elements, 1 metal and 1 non metal. their name is always written as 2 element names, plus the ide suffix attached to the second name. [1] examples of simple binary ionic compounds include potassium oxide and sodium phosphide. This chemistry video tutorial provides an introduction to writing the formula of an ionic compound that contains transition metals with roman numerals and po.

Writing Ionic Formulas Basic Introduction Youtube

Comments are closed.