Hydrogen Spectrum Emission Absorption Series Diagram Vrogue Co
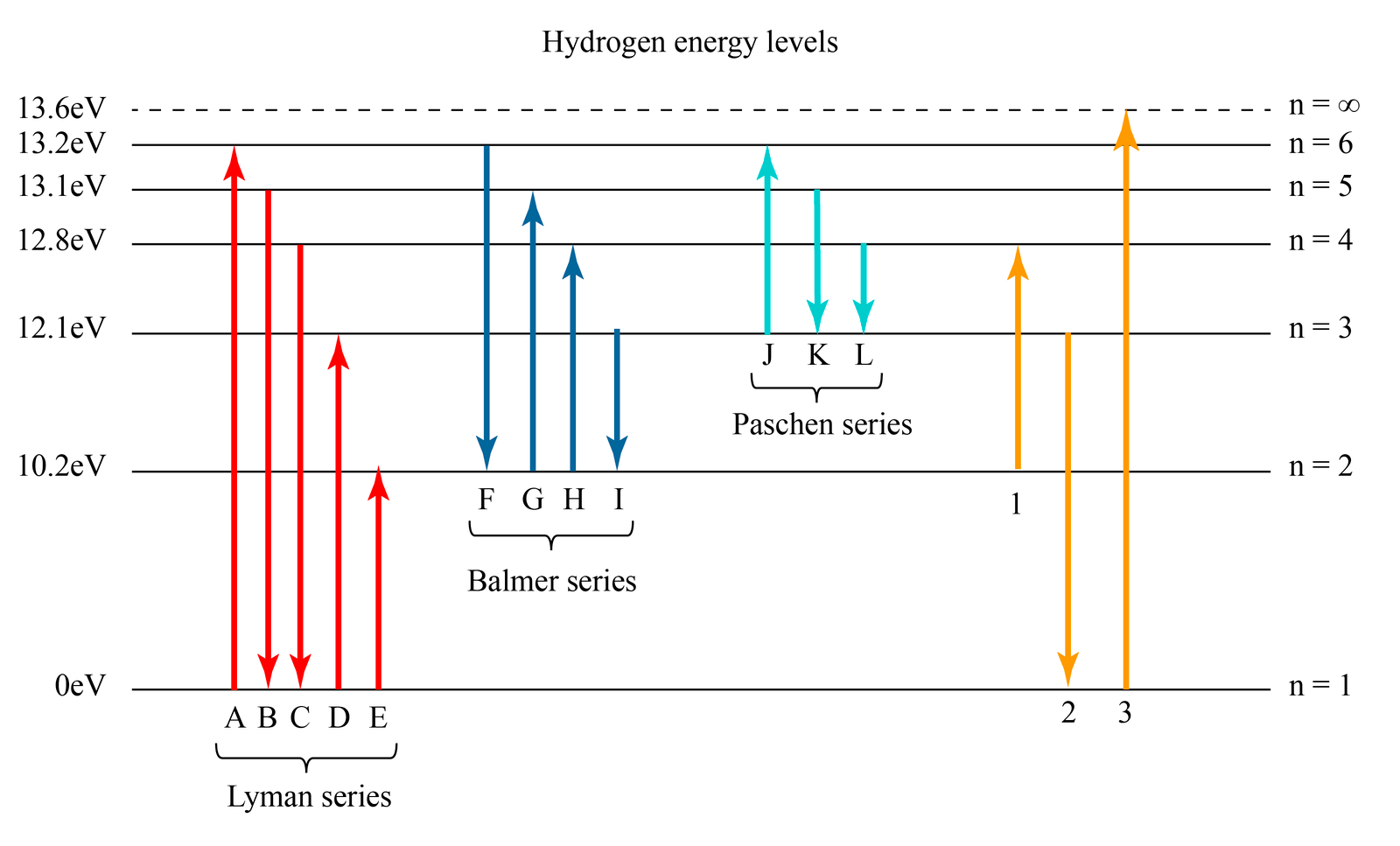
Atomic Spectra Of Hydrogen Atom Rightwith Vrogue Co The spectral series of hydrogen, on a logarithmic scale. the emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the rydberg formula. these observed spectral lines are due to the electron making transitions between two energy levels in an atom. the classification of the series by the. The energy gap between the ground state and the point at which the electron leaves the atom can be determined by substituting the frequency and looking up the value of planck's constant from a data book. Δe = hν = (6.626 × 10 − 34)(3.28 × 1015) = 2.173 × 10 − 18 j. this is the ionization energy for a single atom.

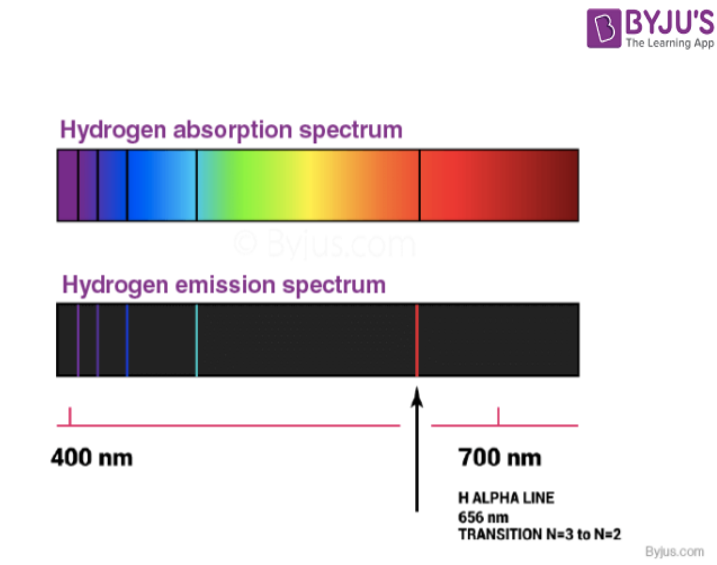
Atomic Spectra Of Hydrogen Atom Rightwith Vrogue Co Figure 7.3.6 absorption and emission spectra. absorption of light by a hydrogen atom. (a) when a hydrogen atom absorbs a photon of light, an electron is excited to an orbit that has a higher energy and larger value of n. (b) images of the emission and absorption spectra of hydrogen are shown here. The spectrum of atomic hydrogen. for almost a century light emitted by the simplest of atoms has been the chief experimental basis for theories of the structure of matter. exploration of the hydrogen spectrum continues, now aided by lasers. by theodor w. hansch, arthur l. schawlow and george w. series. Figure 1.4.1 shows two different types of spectra. a continuous spectrum can be produced by an incandescent solid or gas at high pressure (blackbody radiation, for example, is a continuum). an emission spectrum can be produced by a gas at low pressure excited by heat or by collisions with electrons. an absorption spectrum results when light. Four more series of lines were discovered in the emission spectrum of hydrogen by searching the infrared spectrum at longer wave lengths and the ultraviolet spectrum at shorter wavelengths. each of these lines fits the same general equation, where n 1 and n 2 are integers and r h is 1.09678 x 10 2 nm 1.
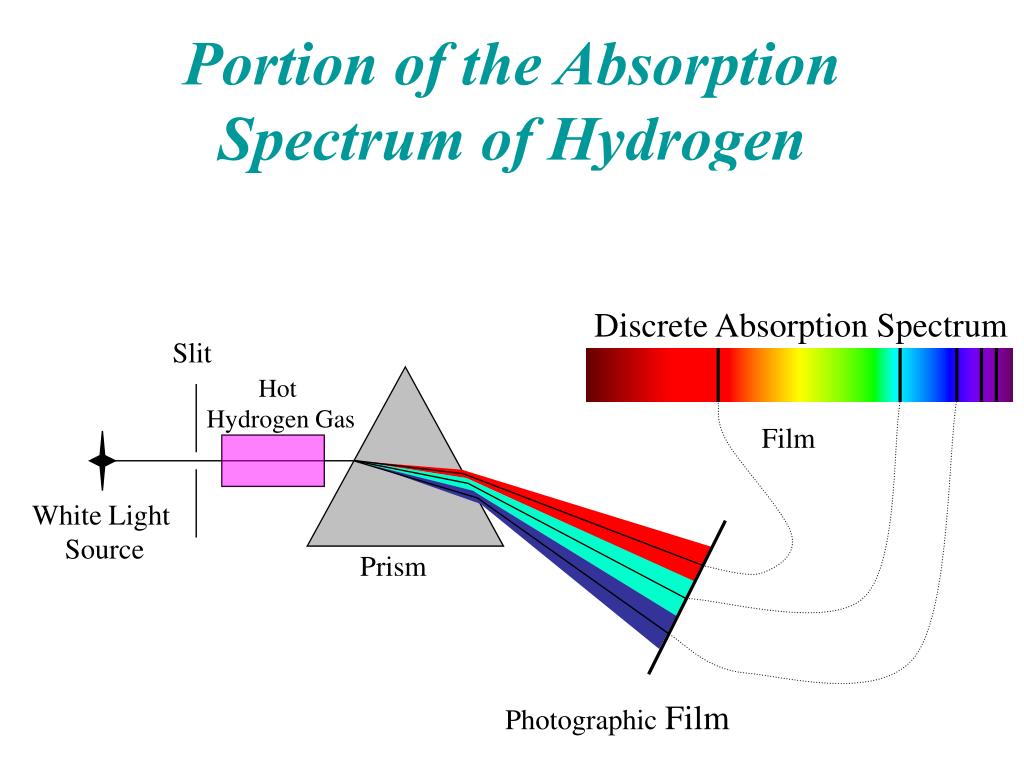
Hydrogen Spectrum Emission Absorption Series Diagram Vrogue Co Figure 1.4.1 shows two different types of spectra. a continuous spectrum can be produced by an incandescent solid or gas at high pressure (blackbody radiation, for example, is a continuum). an emission spectrum can be produced by a gas at low pressure excited by heat or by collisions with electrons. an absorption spectrum results when light. Four more series of lines were discovered in the emission spectrum of hydrogen by searching the infrared spectrum at longer wave lengths and the ultraviolet spectrum at shorter wavelengths. each of these lines fits the same general equation, where n 1 and n 2 are integers and r h is 1.09678 x 10 2 nm 1. The difference between the absorption spectrum and the emission spectrum is explained in figure 6.14. an absorption spectrum is observed when light passes through a gas. this spectrum appears as black lines that occur only at certain wavelengths on the background of the continuous spectrum of white light (figure 6.13). the missing wavelengths. Hydrogen molecules are first broken up into hydrogen atoms (hence the atomic hydrogen emission spectrum) and electrons are then promoted into higher energy levels. suppose a particular electron was excited into the third energy level. this would tend to lose energy again by falling back down to a lower level.
/GettyImages-1096547948-35b3799817ca4b2fa06888893ef4a348.jpg)
Hydrogen Spectrum Emission Absorption Series Diagram Vrogue Co The difference between the absorption spectrum and the emission spectrum is explained in figure 6.14. an absorption spectrum is observed when light passes through a gas. this spectrum appears as black lines that occur only at certain wavelengths on the background of the continuous spectrum of white light (figure 6.13). the missing wavelengths. Hydrogen molecules are first broken up into hydrogen atoms (hence the atomic hydrogen emission spectrum) and electrons are then promoted into higher energy levels. suppose a particular electron was excited into the third energy level. this would tend to lose energy again by falling back down to a lower level.
Hydrogen Spectrum Emission Absorption Series Diagram Vrogue Co

Comments are closed.