Ind Data Requirements And Us Fda Submission Process
Ind Data Requirements And Us Fda Submission Process Electronic submissions should be considered whenever possible (fda study data standards resources). each application should be accompanied by: form 1571 (pdf 830kb) (ind application cover),. The us fda submission process is a critical step to ensure the safety and efficacy of new drugs. to ensure compliance with the fda, drug developers must have an effective data strategy in place. this includes understanding the ind data requirements and having the plan to collect, analyze, and submit all relevant information to the fda.
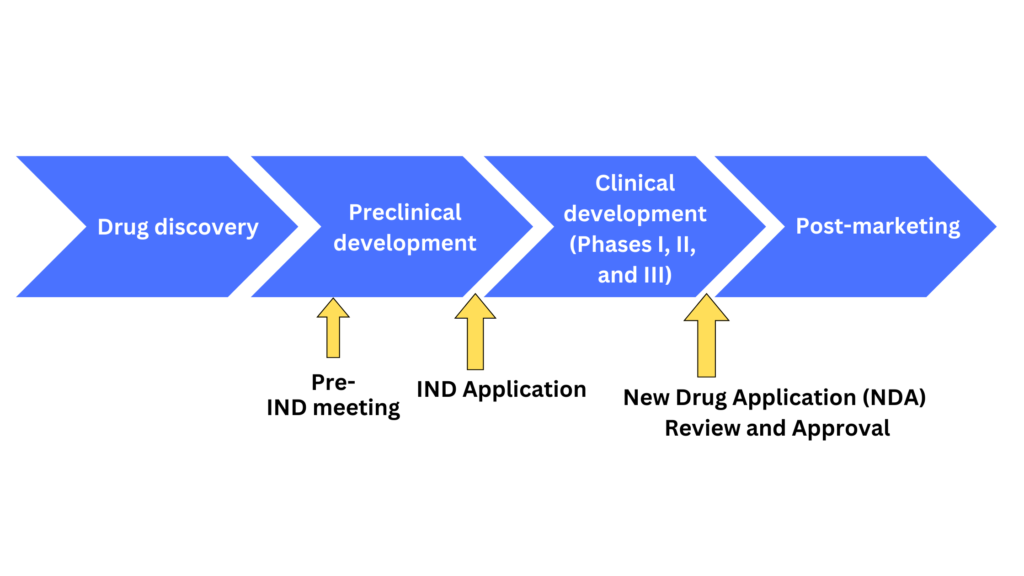
Ind Data Requirements And Us Fda Submission Process Instructions for sponsors of emergency investigational new drug (eind) applications for antimicrobial products. from the office of antimicrobial products, division of antiviral products. emergency. Drug master files (dmfs) dmf submission resources. 02 15 2022. forms & submission requirements and applications. An investigational new drug offered for import into the united states complies with the requirements of this part if it is subject to an ind that is in effect for it under § 312.40 and: (1) the consignee in the united states is the sponsor of the ind; (2) the consignee is a qualified investigator named in the ind; or. The ind submission stage is a critical step in the fda approval process. a successful submission requires careful planning, preparation, and attention to detail. sponsors must ensure their ind application is complete, correct, and meets the fda’s data quality and compliance standards.

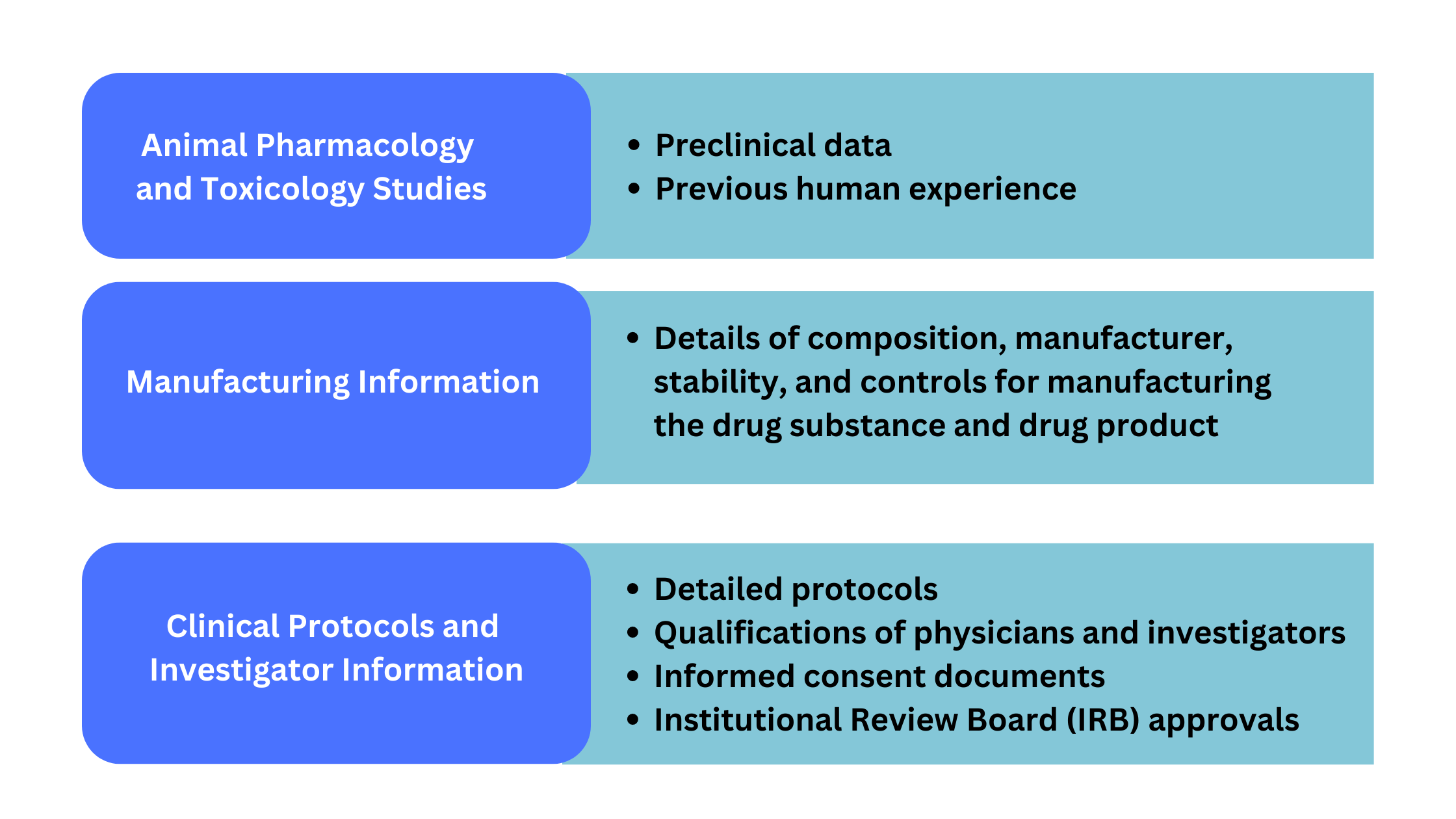
Ind Data Requirements And Us Fda Submission Process An investigational new drug offered for import into the united states complies with the requirements of this part if it is subject to an ind that is in effect for it under § 312.40 and: (1) the consignee in the united states is the sponsor of the ind; (2) the consignee is a qualified investigator named in the ind; or. The ind submission stage is a critical step in the fda approval process. a successful submission requires careful planning, preparation, and attention to detail. sponsors must ensure their ind application is complete, correct, and meets the fda’s data quality and compliance standards. The fda web site has downloadable forms, descriptions of the ind application process, and listings of guidance on the completion of the forms and clerical requirements. 13 the fda has issued a guidance that addresses the ind submission process specifically for sponsor investigators. 14 an extensive information for sponsors to guide preclinical. A successful ind application has thorough information on the biological, physical and chemical characteristics of the drug, including all of the ingredients and their purpose. [1] it's critical to support the stability of the drug in this section and provide information that proves the compound's intended effect materializes.

Comments are closed.