Introduction To Fractional Distillation Distillation Procedure Home

Introduction To Fractional Distillation Distillation Procedure Home 👉 to access the full video, please call: 8010963963introduction to fractional distillation | distillation procedure| home revise | chemistry experiment👉 in. Droplets of liquid should be seen in the fractional column, but there should never be a large pool of liquid (flooding). if the column floods, allow the liquid to drain back into the distilling flask and heat at a gentler rate. this page titled 1.2.3.4: step by step procedures for fractional distillation is shared under a cc by nc nd 4.0.

Fractional Distillation Fractional distillation in a laboratory makes use of common laboratory glassware and apparatuses, typically including a bunsen burner, a round bottomed flask and a condenser, as well as the single purpose fractionating column. fractional distillation. as an example, consider the distillation of a mixture of water and ethanol. ethanol boils at. 5.3: fractional distillation a simple distillation is incapable of significant purification if the boiling points of the components are too close. when the difference in boiling points is less than 100 ˚c, a modification is necessary, namely insertion of a fractionating column between the distilling flask and three way adapter. 5.3a: theory of. Fractional distillation is used when separating mixtures of liquids whose boiling points are more similar (separated by less than 25 °c). this video will detail the fractional distillation of a mixture of two common organic solvents, cyclohexane and toluene. procedure. 1. set up of fractional distillation apparatus. A main distinction between fractional and simple distillation is that the former uses a fractionating column. fractional distillation has multiple real world examples, including the fractional distillation of air. fractional distillation produces fractions; these can be pure or mixtures of chemicals with very similar boiling points.
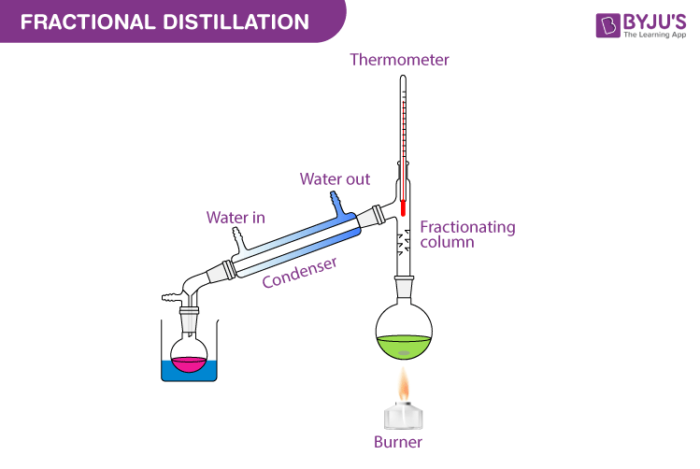
Fractional Distillation Detailed Explanation Along With Diagrams Fractional distillation is used when separating mixtures of liquids whose boiling points are more similar (separated by less than 25 °c). this video will detail the fractional distillation of a mixture of two common organic solvents, cyclohexane and toluene. procedure. 1. set up of fractional distillation apparatus. A main distinction between fractional and simple distillation is that the former uses a fractionating column. fractional distillation has multiple real world examples, including the fractional distillation of air. fractional distillation produces fractions; these can be pure or mixtures of chemicals with very similar boiling points. Preparation for the distillation. remove the round bottom flask from the assembly by lowering it to the base of the retort stand. place a stemmed funnel into the top of the round bottom flask, and add the liquid to be distilled. do not fill the flask more than half full. after filling the flask, remove the funnel. Fractional distillation is a key process in refining crude oil into various petroleum products. 2. chemical industry: applied in the separation and purification of chemicals and solvents. 3. alcohol production: used in the production of alcoholic beverages to separate ethanol from other components. 4. pharmaceuticals:.

Fractional Distillation Diagram Labeled Preparation for the distillation. remove the round bottom flask from the assembly by lowering it to the base of the retort stand. place a stemmed funnel into the top of the round bottom flask, and add the liquid to be distilled. do not fill the flask more than half full. after filling the flask, remove the funnel. Fractional distillation is a key process in refining crude oil into various petroleum products. 2. chemical industry: applied in the separation and purification of chemicals and solvents. 3. alcohol production: used in the production of alcoholic beverages to separate ethanol from other components. 4. pharmaceuticals:.
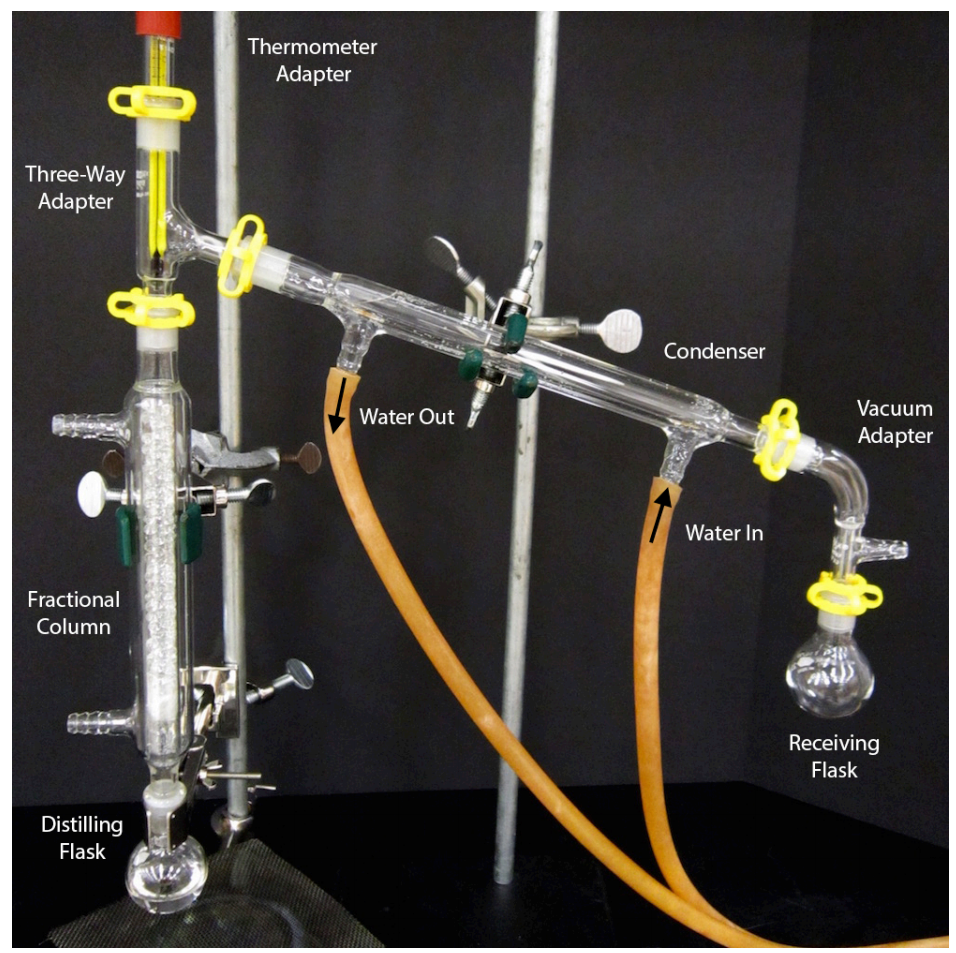
5 3d Step By Step Procedures For Fractional Distillation Chemistry

Comments are closed.