Knowledge Sea Atomic Spectrum

Knowledge Sea Atomic Spectrum Atomic spectrum. the light which atoms give off is made up of specific wavelengths, called lines; observed by a spectroscope, the lines are, collectively, atomic spectra. in an atom, electrons have specific and discrete energies. there are many more energy states (or levels) in each atom than there are electrons. when an electron transitions. This page titled 4.2: understanding atomic spectra is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by elizabeth gordon. the ground state of an atom is the lowest energy state of the atom. when those atoms are given energy, the electrons absorb the energy and move to a higher energy level.

Knowledge Sea Atomic Spectrum Vrogue Co Modified by joshua halpern (howard university) 8.2: atomic spectra is shared under a license and was authored, remixed, and or curated by libretexts. the photoelectric effect provided indisputable evidence for the existence of the photon and thus the particle like behavior of electromagnetic radiation. the concept of the photon, however. 8.2 – atomic spectra. another paradox within the classical electromagnetic theory that scientists in the late nineteenth century struggled with concerned the light emitted from atoms and molecules. when solids, liquids, or condensed gases are heated sufficiently, they radiate some of the excess energy as light. The swedish physicist johannes rydberg (1854–1919) subsequently restated and expanded balmer’s result in the rydberg equation: 1 λ = r (1 n21 − 1 n22) (2.3.2) (2.3.2) 1 λ = ℜ (1 n 1 2 − 1 n 2 2) . where n1 and n2 are positive integers, n2 > n1, and r ℜ the rydberg constant, has a value of 1.09737 × 10 7 m −1. Atomic spectra are defined as. the spectrum of the electromagnetic radiation emitted or absorbed by an electron during transitions between different energy levels within an atom. when an electron gets excited from one energy level to another, it either emits or absorbs light of a specific wavelength. the collection of all these specific.
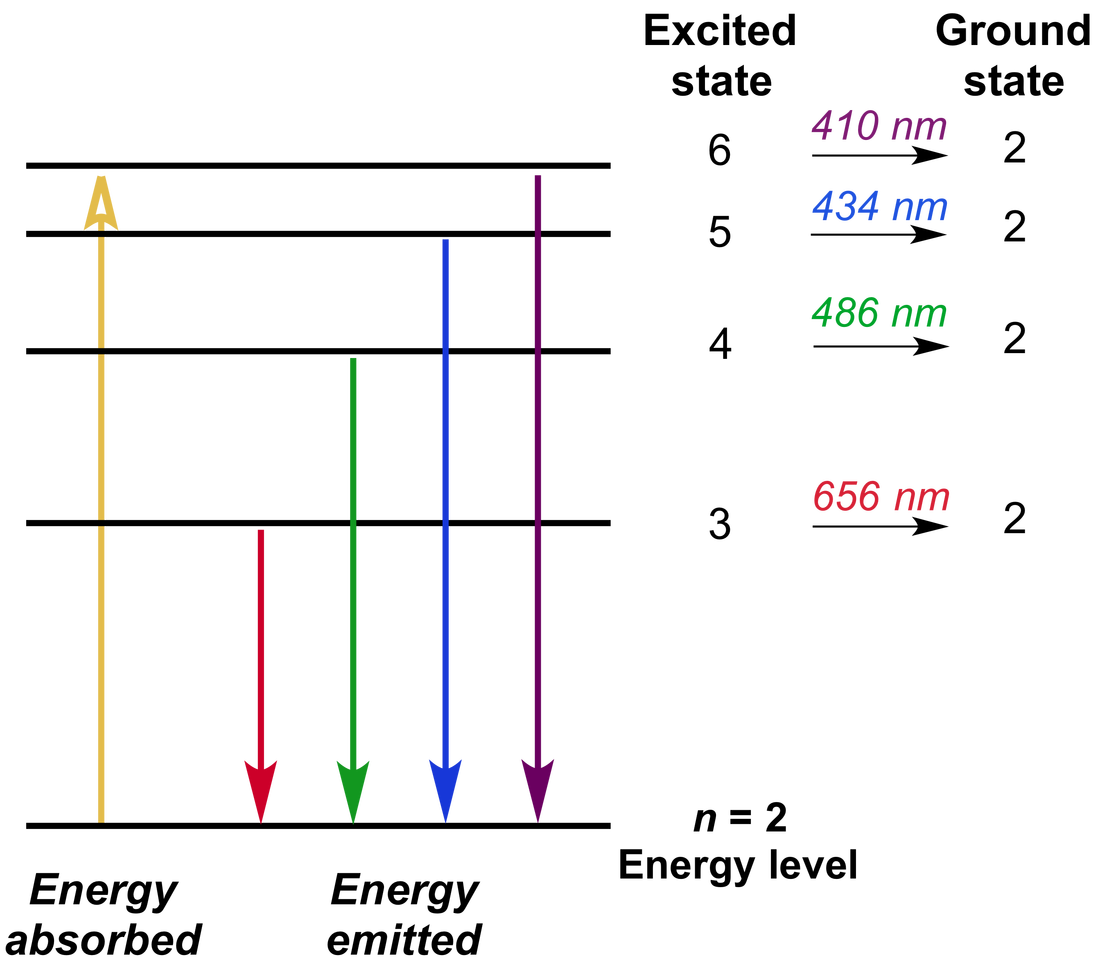
Atomic Spectra And Its Types The swedish physicist johannes rydberg (1854–1919) subsequently restated and expanded balmer’s result in the rydberg equation: 1 λ = r (1 n21 − 1 n22) (2.3.2) (2.3.2) 1 λ = ℜ (1 n 1 2 − 1 n 2 2) . where n1 and n2 are positive integers, n2 > n1, and r ℜ the rydberg constant, has a value of 1.09737 × 10 7 m −1. Atomic spectra are defined as. the spectrum of the electromagnetic radiation emitted or absorbed by an electron during transitions between different energy levels within an atom. when an electron gets excited from one energy level to another, it either emits or absorbs light of a specific wavelength. the collection of all these specific. Atomic spectra 3 i 1. the determination of atomic (or molecular) energy levels. a knowledge of the atomic energy levels is a prerequisite for understanding many as pects of chemical reactivity and structure. furthermore, the energy levels can be used to test quantum theories and gain a fundamental understand ing of the underlying physics. 2. Book chapters topics 6.3, “atomic spectra and models of the atom.” : line spectra; the bohr model; uses of emission and absorption spectra : lecture video. view video page. download video; download transcript; resources. lecture slides (pdf 1.3mb). lecture summary. this lecture gives more details about the atomic spectra of hydrogen along with matter energy interactions involving atomic.

Knowledge Sea Atomic Spectrum Vrogue Co Atomic spectra 3 i 1. the determination of atomic (or molecular) energy levels. a knowledge of the atomic energy levels is a prerequisite for understanding many as pects of chemical reactivity and structure. furthermore, the energy levels can be used to test quantum theories and gain a fundamental understand ing of the underlying physics. 2. Book chapters topics 6.3, “atomic spectra and models of the atom.” : line spectra; the bohr model; uses of emission and absorption spectra : lecture video. view video page. download video; download transcript; resources. lecture slides (pdf 1.3mb). lecture summary. this lecture gives more details about the atomic spectra of hydrogen along with matter energy interactions involving atomic.

Knowledge Sea Atomic Spectrum Vrogue Co

Comments are closed.