Lab 3 Serial Dilution Calculations

Infographicвђ Lab Basics How To Perform Serial Dilutions Carolina The serial dilution method was first described in 1883 by german scientist and physician robert koch when he published his work on infectious disease causing agents,1 and is now a standard technique in today's laboratories. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. the stock solution of naoh is 10%. draw diagram as part of your description. using the standard curve below, calculate the concentration of an unknown solution if its absorbance is 0.55. figure 3.
Serial Dilutions Lab At Joseph Brown Blog Procedure of serial dilution. the following is the procedure for a ten fold dilution of a sample to a dilution factor of 10 6:. the sample culture is taken in a test tube and six test tubes, each with 9 ml of sterile diluents, which can either be distilled water or 0.9% saline, are taken. You take 10% off from each of the serial dilutions (i.e., a 1:10 dilution factor) and, after 4 dilutions, have a solution with approximately 10 cells. with this known number, you can get a better understanding of the effectiveness of your drug. doctors and nurses also often use serial dilutions. The procedure for how to perform serial dilutions (e.g. 5 fold) is illustrated in figure 2 and divided into the following steps: distribution of 80 µl of diluent (water) to the 96 well flat bottom plates. transfer of 20 µl of highly concentrated tartrazine into the first column. mixing. A serial dilution is a sequence of dilutions created using the same dilution factor. for instance, creating a two fold dilution with a starting concentration of 10 µm yields the following concentrations: 10 µm, 5 µm, 2.5 µm, 1.25 µm, etc. the tool below can be used to create a protocol for preparing a serial dilution from a stock solution.

Serial Dilution Method Protocol Step Wise Explanation Youtube The procedure for how to perform serial dilutions (e.g. 5 fold) is illustrated in figure 2 and divided into the following steps: distribution of 80 µl of diluent (water) to the 96 well flat bottom plates. transfer of 20 µl of highly concentrated tartrazine into the first column. mixing. A serial dilution is a sequence of dilutions created using the same dilution factor. for instance, creating a two fold dilution with a starting concentration of 10 µm yields the following concentrations: 10 µm, 5 µm, 2.5 µm, 1.25 µm, etc. the tool below can be used to create a protocol for preparing a serial dilution from a stock solution. Serial dilutions are often performed in steps of 10 or 100. they are described as ratios of the initial and final concentrations. for example, a 1:10 dilution is a mixture of one part of a solution and nine parts fresh solvent. for a 1:100 dilution, one part of the solution is mixed with 99 parts new solvent. Serial dilution in microbiology step by step. step 1: transfer 1g or 1 ml of specimen or sample into 9ml of normal saline solution dilution to become 10 1 solution. step 2: transfer 1 ml of 10 1 solution to the next tube which contains 9 ml of saline solution and it becomes 10 2 solution. step 3: repeat this process for successive dilution and.
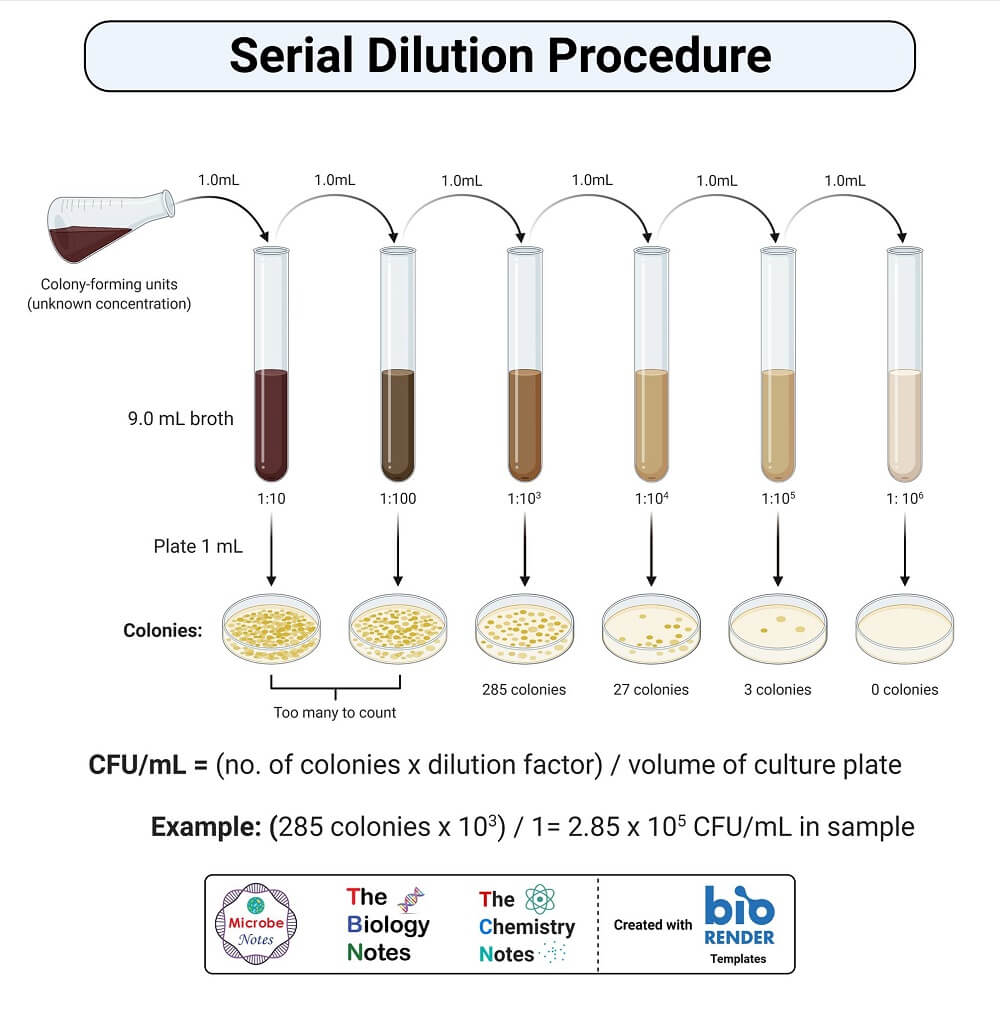
Serial Dilution Formula Calculator Method Uses Examples Serial dilutions are often performed in steps of 10 or 100. they are described as ratios of the initial and final concentrations. for example, a 1:10 dilution is a mixture of one part of a solution and nine parts fresh solvent. for a 1:100 dilution, one part of the solution is mixed with 99 parts new solvent. Serial dilution in microbiology step by step. step 1: transfer 1g or 1 ml of specimen or sample into 9ml of normal saline solution dilution to become 10 1 solution. step 2: transfer 1 ml of 10 1 solution to the next tube which contains 9 ml of saline solution and it becomes 10 2 solution. step 3: repeat this process for successive dilution and.

Comments are closed.