Moderna Completes Full Fda Approval Request Of Covid Vaccine Pfizer Seeks 3rd Dose Approval
Three Covid 19 Vaccines Compared Pfizer Moderna Johnson Johnson The updated mrna covid 19 vaccines include comirnaty and spikevax, both of which are approved for individuals 12 years of age and older, and the moderna covid 19 vaccine and pfizer biontech covid. Moderna announced on wednesday it has completed its submission to the us food and drug administration for full approval of its covid 19 vaccine for people age 18 and older, and pfizer and biontech.
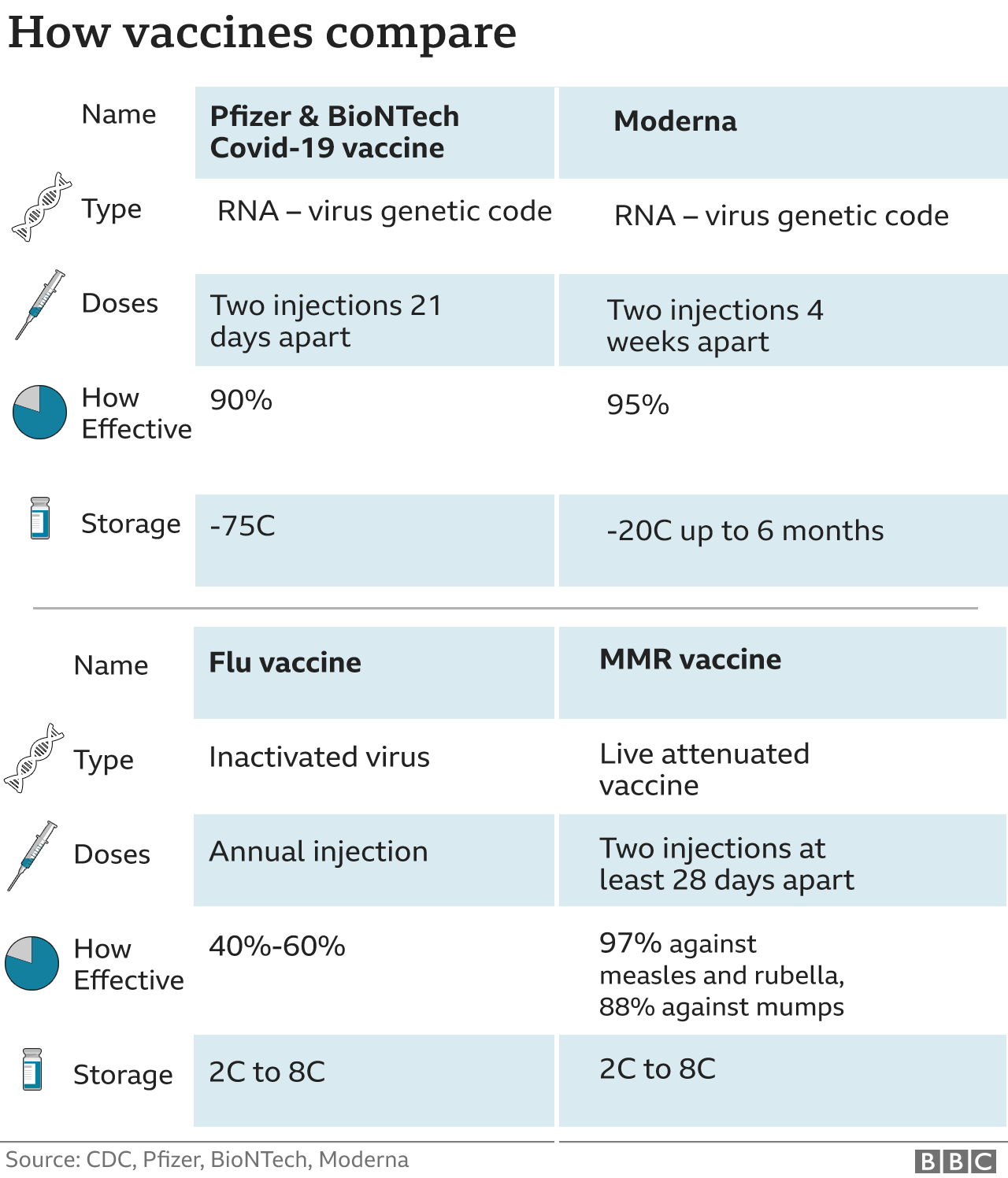
Covid Us Approves Moderna As Second Vaccine Bbc News In june 2023, the u.s. fda advised that covid 19 vaccines should be updated to a monovalent xbb.1.5 composition for the 2023 2024 vaccination season. at the june vrbpac, moderna presented clinical data showing that its updated vaccine resulted in robust immune responses across multiple xbb sublineages, including xbb.1.5 and xbb.1.16. The vaccine, called spikevax, is the second to receive full fda approval in the united states, joining pfizer's vaccine. a health worker administers a dose of the moderna covid 19 vaccine in. The fda amended the emergency use authorizations (eua) for both the moderna and pfizer biontech covid 19 vaccines authorizing use of a single booster dose for all individuals 18 years of age and. A look at the latest guidance from the fda. the food and drug administration on monday granted pfizer and biontech full u.s. approval of their covid 19 vaccine – becoming the first in the u.s.

Comments are closed.