New Antibody Treatment To Protect Against Malaria Shows 88 Percent
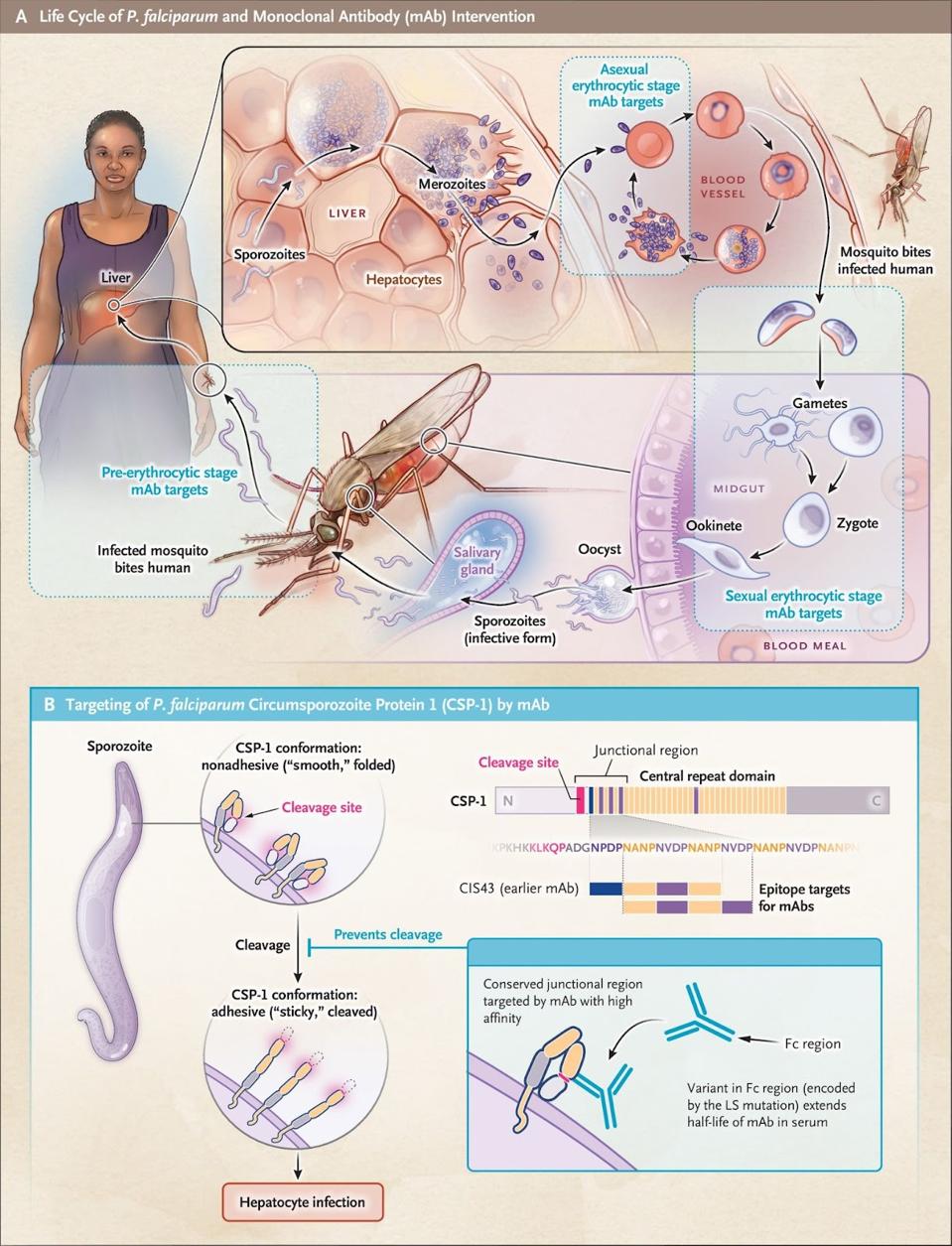
New Monoclonal Antibody For Treatment Of Malaria вђ Access Over a 6 month malaria season, this monoclonal antibody provided protective efficacy against p. falciparum infection of 75.0% and 88.2% at the doses of 10 mg per kilogram and 40 mg per kilogram. The antibody, known as cis43ls, was up to 88% effective at preventing against infection from plasmodium falciparum malaria over the course of a six month intense malaria season. the trial involved.
New Monoclonal Antibody For Treatment Of Malaria вђ Access In a phase 3 trial, rts,s as01 showed 36% efficacy against clinical malaria over a period of 4 years in children 5 to 17 months of age who had received four doses. 5 until vaccines that induce. Malaria is a mosquito borne parasitic disease caused by plasmodium falciparum that affects approximately 200 to 400 million people each year, resulting in nearly 400,000 annual deaths and. Recent reports show that treatment with mabs 10 can completely prevent malaria following controlled human infection 11 and provide 88% efficacy (prevention of infection) for 6 months in endemic. Clinical trials are now underway in mali and kenya, where malaria is widespread. researchers are testing to see if injections of l9ls can protect infants and young children from malaria for up to 12 months. “this is the first demonstration that a monoclonal antibody can provide protection with a low dose given by the subcutaneous route.

New Antibody Treatment To Protect Against Malaria Shows 88 Percent Recent reports show that treatment with mabs 10 can completely prevent malaria following controlled human infection 11 and provide 88% efficacy (prevention of infection) for 6 months in endemic. Clinical trials are now underway in mali and kenya, where malaria is widespread. researchers are testing to see if injections of l9ls can protect infants and young children from malaria for up to 12 months. “this is the first demonstration that a monoclonal antibody can provide protection with a low dose given by the subcutaneous route. University of maryland school of medicine researchers found cis43ls to be safe, well tolerated in human challenge trial. a monoclonal antibody treatment was found to be safe, well tolerated, and effective in protecting against malaria in a small group of healthy volunteers who were exposed to malaria in a challenge study, according to new research published in the lancet infectious diseases by. One injection of a candidate monoclonal antibody (mab) known as l9ls was found to be safe and highly protective in u.s. adults exposed to the malaria parasite, according to results from a national institutes of health phase 1 clinical trial published in the new england journal of medicine.

Comments are closed.