Pdf Systematic Review Of Guidelines For Internal Validity In The

Pdf Systematic Review Of Guidelines For Internal Validity In The Areas covered: in this review, the authors summarize the literature available on different strategies to improve external validity in in vivo, in vitro, or ex vivo experiments; systematic. A systematic review of preclinical research guidelines and organized recommendations according to the type of validity threat (internal, construct, or external) or programmatic research activity they primarily address provided a starting point for developing preclinical guidelines in other disease domains.
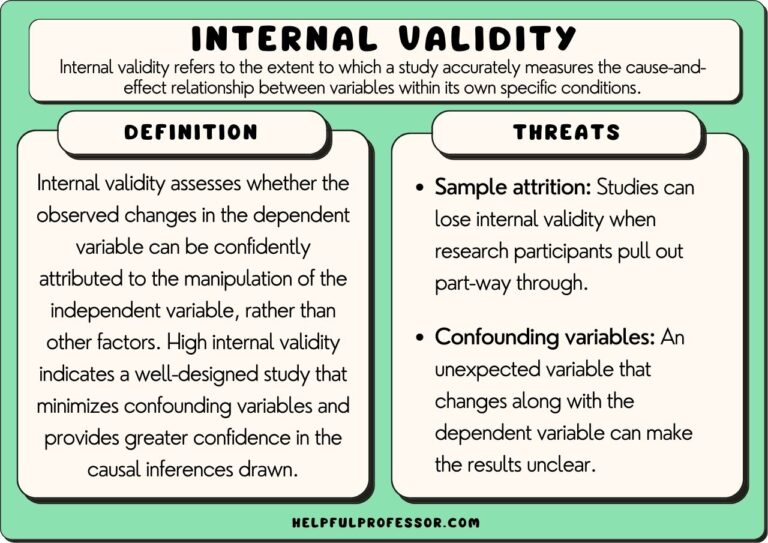
11 Internal Validity Examples 2024 Reassessing the goals of this review, we decided to focus on internal validity, in the protocol we used the term ‘internal validity and reproducibility’. in the protocol, we mention that the aim of this systematic review is an effort to harmonise guidelines and create a unified framework. this is still under way and will be published. Appraising systematic reviews helps to identify potential. sources of bias or limitations in the review process and. can help to guide future research in the field ( page et al., 2021. Systematic review will affect the confidence we can have in the results of the systematic review. see resources listed in appendix a of this guide for further information on quality and validity. generally, assessment of study quality includes assessment of at least some elements of the internal validity of the study4. this is the degree to. Request pdf | on apr 15, 2023, esther schenker published systematic review of guidelines for internal validity in the design, conduct and analysis of preclinical biomedical experiments involving.
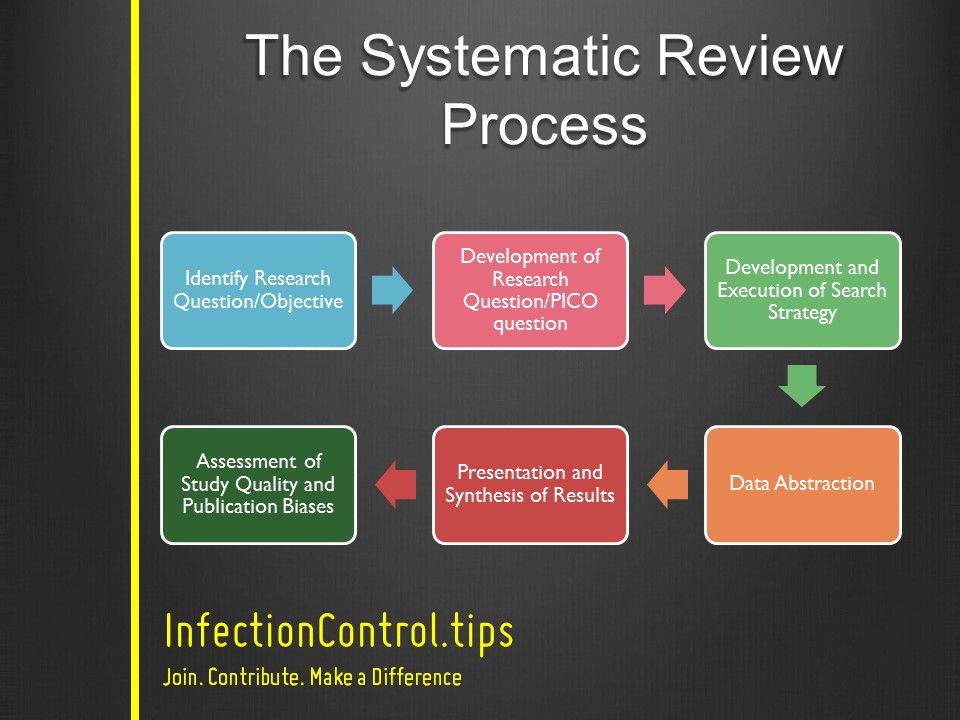
The Systematic Review Process Infectioncontrol Tips Systematic review will affect the confidence we can have in the results of the systematic review. see resources listed in appendix a of this guide for further information on quality and validity. generally, assessment of study quality includes assessment of at least some elements of the internal validity of the study4. this is the degree to. Request pdf | on apr 15, 2023, esther schenker published systematic review of guidelines for internal validity in the design, conduct and analysis of preclinical biomedical experiments involving. Vollert j, schenker e, macleod m, bespalov a, wuerbel h, michel m, dirnagl u, potschka h, waldron am, wever k, steckler t, van de casteele t, altevogt b, sil a, rice asc, the eqipd wp3 study group members (2020) systematic review of guidelines for internal validity in the design, conduct and analysis of preclinical biomedical experiments. The internal validity of conclusions about effectiveness or impact in systematic reviews, and of decisions based on them, depends on risk of bias assessments being conducted appropriately. however, a random sample of 50 recently published articles claiming to be quantitative environmental systematic reviews found 64% did not include any risk of bias assessment, whilst nearly all that did.

Guidance On Conducting A Systematic Literature Review Yu Xiao Maria Vollert j, schenker e, macleod m, bespalov a, wuerbel h, michel m, dirnagl u, potschka h, waldron am, wever k, steckler t, van de casteele t, altevogt b, sil a, rice asc, the eqipd wp3 study group members (2020) systematic review of guidelines for internal validity in the design, conduct and analysis of preclinical biomedical experiments. The internal validity of conclusions about effectiveness or impact in systematic reviews, and of decisions based on them, depends on risk of bias assessments being conducted appropriately. however, a random sample of 50 recently published articles claiming to be quantitative environmental systematic reviews found 64% did not include any risk of bias assessment, whilst nearly all that did.
Pdf Usfda Guidelines On Process Validation A Review

Comments are closed.