Periodic Table Explained Introduction
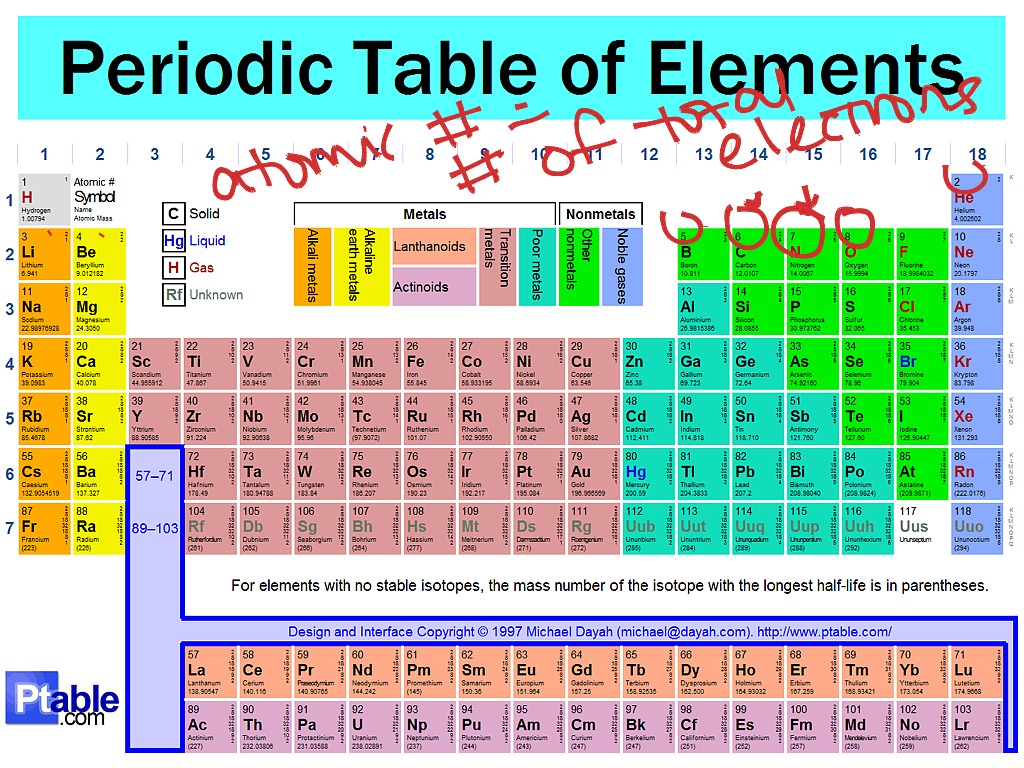
Periodic Table Elements Explained Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number —i.e., the total number of protons in the atomic nucleus. when the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group. Follow us at facebook atomicschool, instagram atomicschools and twitter atomicschoolscheck out how the the perio.

Periodic Table Explained Introduction Periodic Table Periodic Table Im A more comprehensive description of the periodic table is found in chapter 7. figure 1.8.1 1.8. 1 the periodic table showing the elements in order of increasing z. the metals are on the bottom left in the periodic table, and the nonmetals are at the top right. the semimetals lie along a diagonal line separating the metals and nonmetals. The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods ") and columns (" groups "). it is an icon of chemistry and is widely used in physics and other sciences. it is a depiction of the periodic law, which states that when the elements are arranged in order. The periodic table lists elements by atomic number, which is the number of protons in every atom of that element. atoms of an atomic number may have varying numbers of neutrons (isotopes) and electrons (ions), yet remain the same chemical element. elements in the periodic table are arranged in periods (rows) and groups (columns). Introduction to the periodic table. people have known about elements like carbon and gold since ancient time. the elements couldn't be changed using any chemical method. each element has a unique number of protons. if you examine samples of iron and silver, you can't tell how many protons the atoms have. however, you can tell the elements apart.

Periodic Table Explained Introduction Youtube The periodic table lists elements by atomic number, which is the number of protons in every atom of that element. atoms of an atomic number may have varying numbers of neutrons (isotopes) and electrons (ions), yet remain the same chemical element. elements in the periodic table are arranged in periods (rows) and groups (columns). Introduction to the periodic table. people have known about elements like carbon and gold since ancient time. the elements couldn't be changed using any chemical method. each element has a unique number of protons. if you examine samples of iron and silver, you can't tell how many protons the atoms have. however, you can tell the elements apart. The chemistry of each element is determined by its number of protons and electrons. in a neutral atom, the number of electrons equals the number of protons. figure 2.8.1 2.8. 1: the periodic table showing the elements in order of increasing z. the metals are on the bottom left in the periodic table, and the nonmetals are at the top right. Periodic table elements, groups, blocks: the periodic table of the elements contains all of the chemical elements that have been discovered or made; they are arranged, in the order of their atomic numbers, in seven horizontal periods, with the lanthanoids (lanthanum, 57, to lutetium, 71) and the actinoids (actinium, 89, to lawrencium, 103) indicated separately below. the periods are of.

Ppt Introduction To The Periodic Table Of Elements Powerpoint The chemistry of each element is determined by its number of protons and electrons. in a neutral atom, the number of electrons equals the number of protons. figure 2.8.1 2.8. 1: the periodic table showing the elements in order of increasing z. the metals are on the bottom left in the periodic table, and the nonmetals are at the top right. Periodic table elements, groups, blocks: the periodic table of the elements contains all of the chemical elements that have been discovered or made; they are arranged, in the order of their atomic numbers, in seven horizontal periods, with the lanthanoids (lanthanum, 57, to lutetium, 71) and the actinoids (actinium, 89, to lawrencium, 103) indicated separately below. the periods are of.
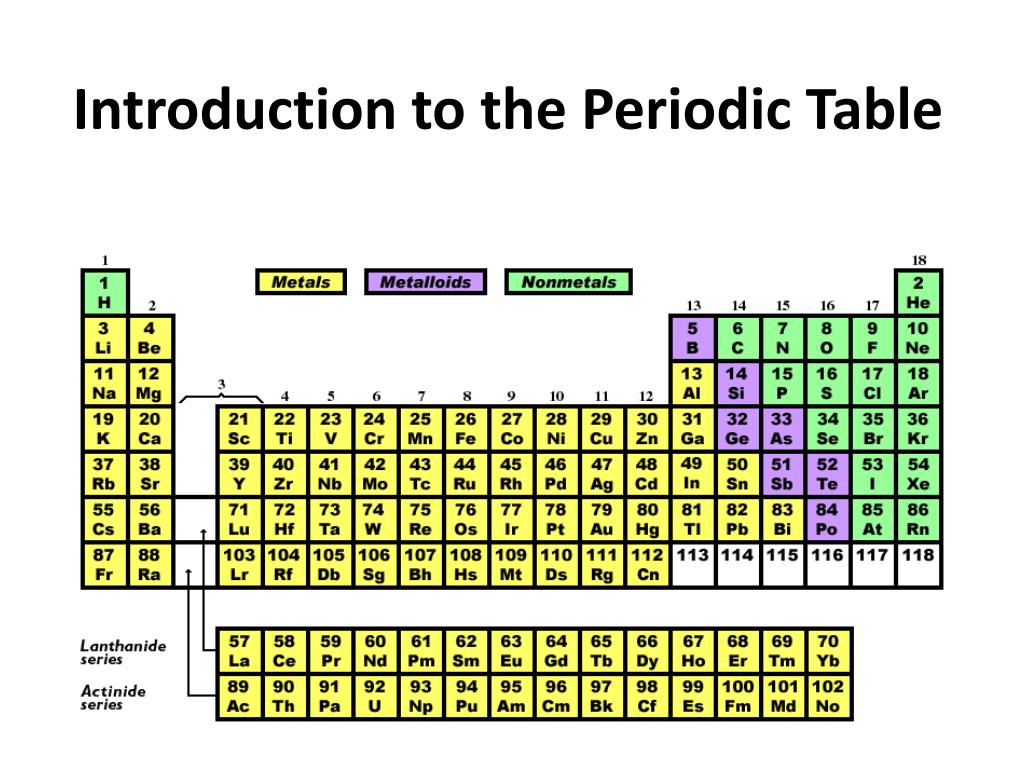
Ppt Introduction To The Periodic Table Powerpoint Presentation Free

Comments are closed.