Periodic Table Families Periodictable Ca
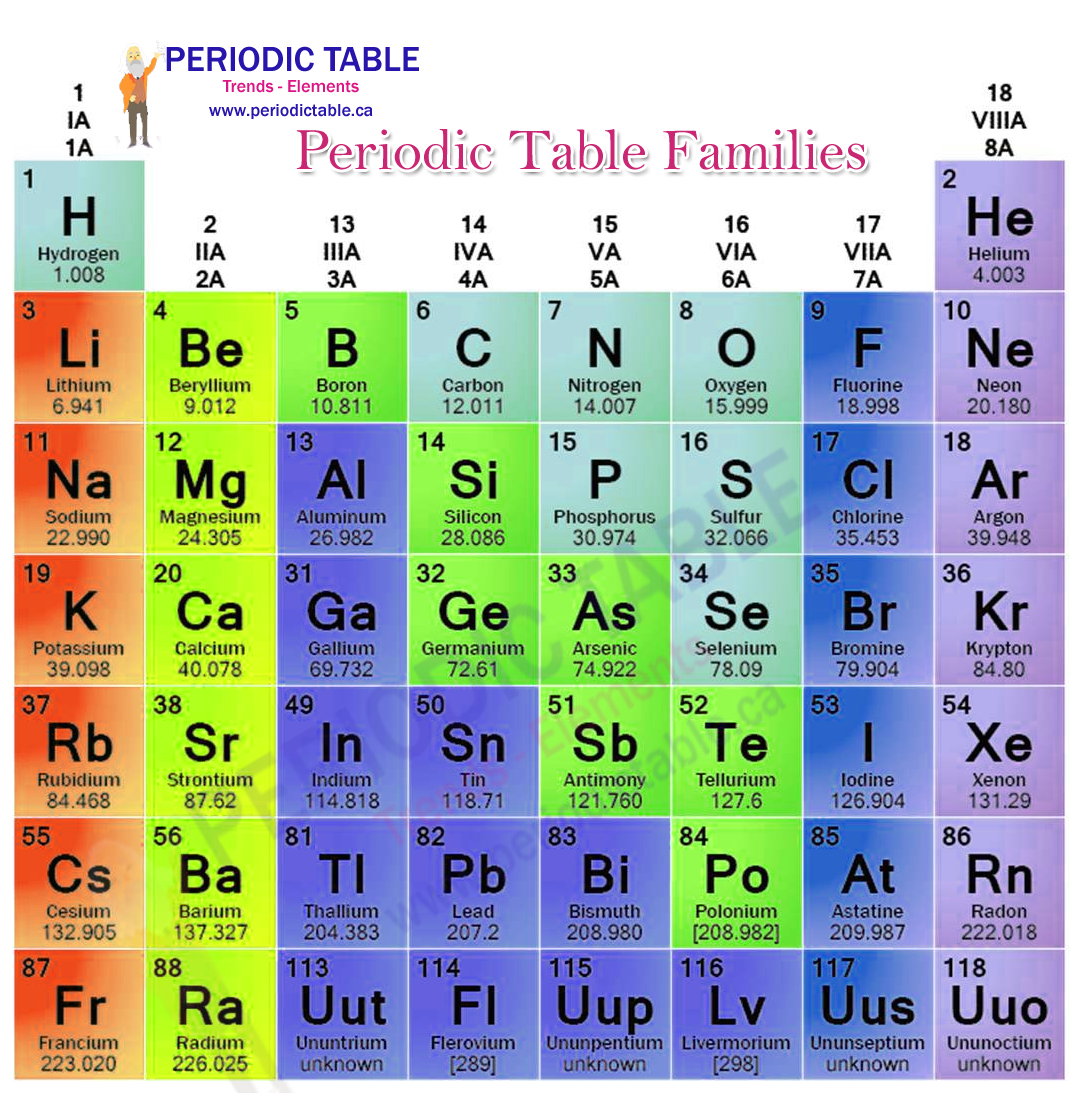
Periodic Table Families Periodictable Ca The periodic table of elements is a way of organising the vast array of different elements found in chemistry. the table is made up of rows, known as periods, and columns, known as groups or families. there are consistent trends as you move around the periodic table of elements, meaning that it is possible to make some very specific predictions. Periods. periods are horizontal rows of the periodic table. they represent elements having the same number of electron shells or energy levels. however, each element has one more proton than its preceding element. thus, the periods are arranged according to the increasing atomic number of the elements. there are seven periods, with the period.
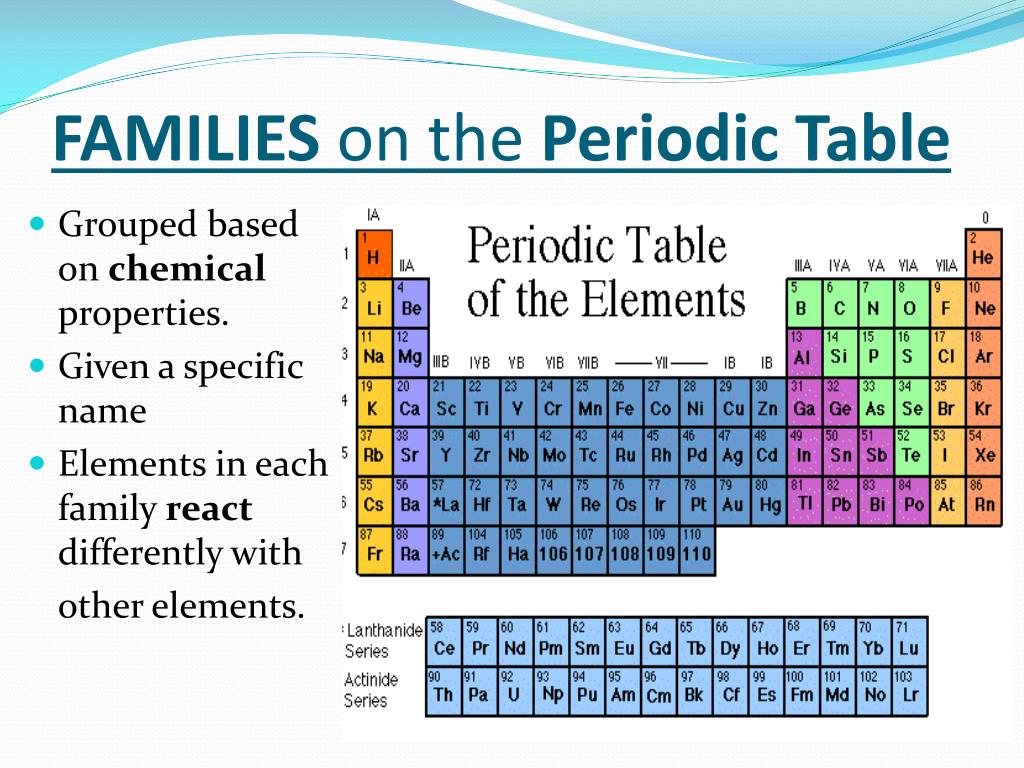
Periodic Table Of Elements Families The periodic table of elements was created in the 1800’s when researchers noted that there were patterns in the reactivity and characteristics of elements when you organised them by atomic mass. the main researcher credited with the creation of the table was mendeleev, but several other researchers were working on similar structures at the. The periodic table of elements provides information on ionic charges. it is possible to estimate the charge that an ion will have from the position the element occupies in the periodic table of elements. elements in group 1 (that is the first column of the periodic table) will usually have ions which have a 1 charge. Calcium (ca) calcium is the 20th element in the periodic table and has a symbol of ca and atomic number of 20. it has an atomic weight of 40.078 and a mass number of 40. calcium has twenty protons and twenty neutrons in its nucleus, and twenty electrons in four shells. it is located in group two, period four and block s of the periodic table. Another common method of categorization recognizes nine element families: alkali metals: group 1 (ia) one valence electron. alkaline earth metals: group 2 (iia) two valence electrons. transition metals: groups 3 12 two valence electrons. boron group or earth metals: group 13 (iiia) three valence electrons.
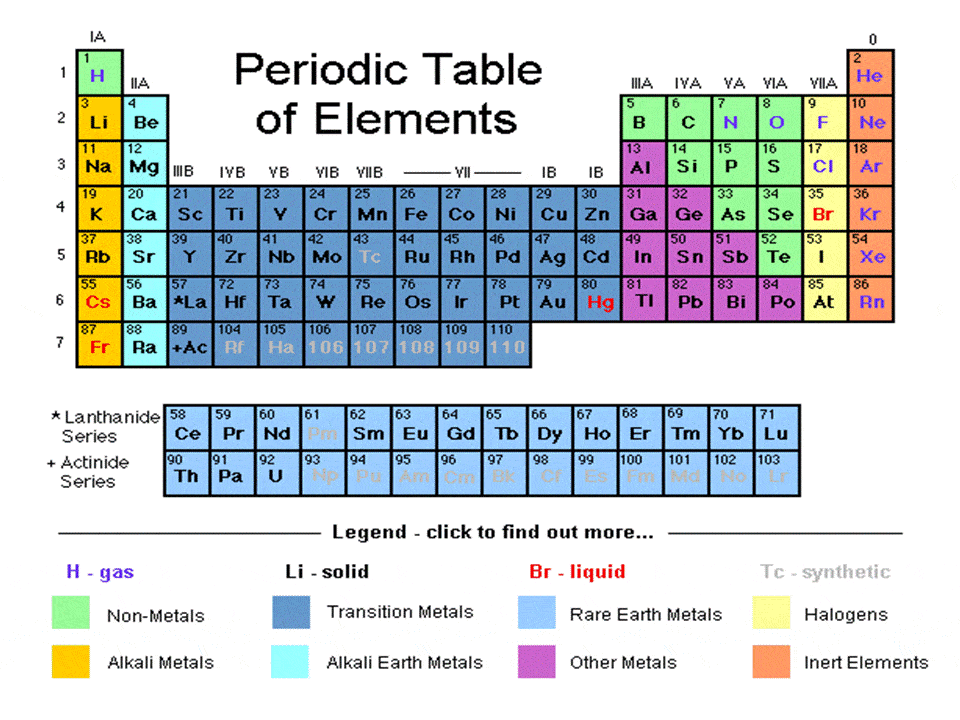
Periodic Table Families Properties Uses Schoolworkhelper Calcium (ca) calcium is the 20th element in the periodic table and has a symbol of ca and atomic number of 20. it has an atomic weight of 40.078 and a mass number of 40. calcium has twenty protons and twenty neutrons in its nucleus, and twenty electrons in four shells. it is located in group two, period four and block s of the periodic table. Another common method of categorization recognizes nine element families: alkali metals: group 1 (ia) one valence electron. alkaline earth metals: group 2 (iia) two valence electrons. transition metals: groups 3 12 two valence electrons. boron group or earth metals: group 13 (iiia) three valence electrons. Interactive periodic table showing names, electrons, and oxidation states. visualize trends, 3d orbitals, isotopes, and mix compounds. 20 ca calcium 40.078; 21 sc. Calcium 40 is a stable isotope containing 20 neutrons. 96.941% of natural calcium is calcium 40. calcium 40 is theorized to actually be a radioactive isotope with an extremely long half life (~10 21 years) based on its internal structure. no one has ever detected a decay of a calcium 40 atom. 42 ca.

Periodic Table Periods Groups And Families Interactive periodic table showing names, electrons, and oxidation states. visualize trends, 3d orbitals, isotopes, and mix compounds. 20 ca calcium 40.078; 21 sc. Calcium 40 is a stable isotope containing 20 neutrons. 96.941% of natural calcium is calcium 40. calcium 40 is theorized to actually be a radioactive isotope with an extremely long half life (~10 21 years) based on its internal structure. no one has ever detected a decay of a calcium 40 atom. 42 ca.

Comments are closed.