Pfizer Biontech Covid 19 Vaccine Gets Full Approval From Fda Opening
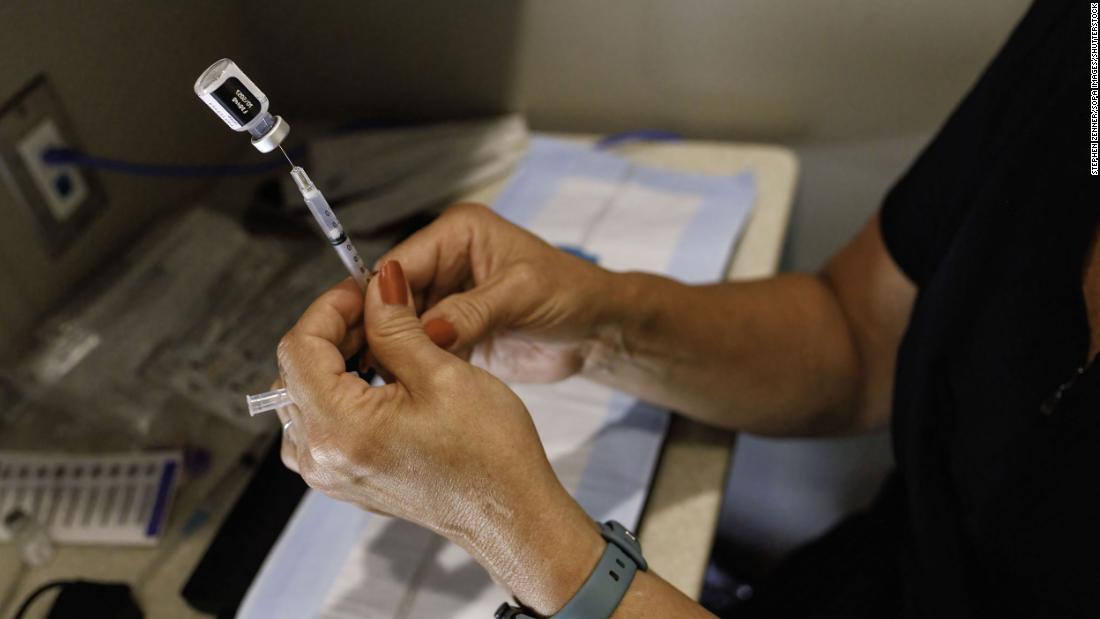
Pfizer Biontech Covid 19 Vaccine Gets Full Approval From Fda Opening 2 This season’s Pfizer and BioNTech COVID-19 vaccine will begin shipping the US beginning in the coming days The approval is based on the full body of previous clinical, non-clinical The FDA said Thursday that people 5 and older are eligible to receive an updated Pfizer or Moderna Covid-19 vaccine as long as it has been at least two months since their last dose Unvaccinated
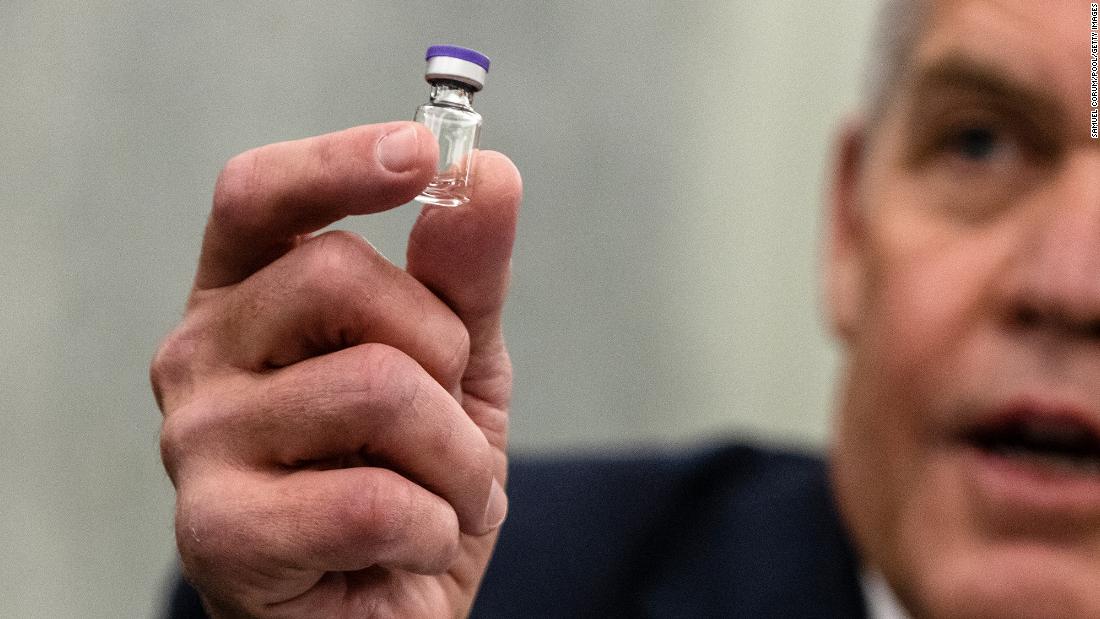
Pfizer Biontech Covid 19 Vaccine Gets Full Approval From Fda Opening The Pfizer/BioNTech bivalent COVID-19 vaccine (Original and Omicron BA4/BA5) has the FDA’s EUA for use in individuals 5 years and over as a single booster dose administered at least two months The US Food and Drug Administration (FDA) has approved updated COVID-19 vaccines from Moderna and Pfizer for the 2024-2025 season The updated mRNA vaccines, Comirnaty and Spikevax, were fully (RTTNews) - Novavax, Inc (NVAX), Friday announced that the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) has received Emergency Use Authorization from the US Food and Drug Moderna said the Spikevax 2024-2025 formula is expected to be available across the US in the days immediately following approval the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID

Pfizer Biontech Covid 19 Vaccine Gets Full Approval From Fda Opening (RTTNews) - Novavax, Inc (NVAX), Friday announced that the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) has received Emergency Use Authorization from the US Food and Drug Moderna said the Spikevax 2024-2025 formula is expected to be available across the US in the days immediately following approval the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID The approval is based on the Please check back for the full information shortly Pfizer-BioNTech COVID-19 Vaccine (2024-2025 Formula)* is FDA authorized under Emergency Use Authorization the US Food and Drug Administration on Thursday signed off on updated Covid-19 vaccines from Moderna and Pfizer/BioNTech Moderna and Pfizer said shots will be available in pharmacies and clinics Pfizer Inc and BioNTech SE announced that the US Food and Drug Administration (FDA) has approved the supplemental Biologics License Application for individuals 12 years of age and older (COMIRNATY

Comments are closed.