Ph And Poh Calculation
Calculation Of Ph And Poh Calculate the ph by rearranging and plugging in the poh: ph = 14.00 – poh. calculate the answers. think about your results. hcl is a strong acid. a ph = 1.00 would be very acidic, so the answer makes sense. ca (oh) 2 is a strong base. a ph = 11.94 would be quite basic, so the answer makes sense. There are two common calculations you will encounter with ph and poh: finding px from [x] (and vice versa) and finding ph knowing poh (or vice versa). calculation of ph from [h 3 o ] what is the ph of stomach acid, a solution of hcl with a hydronium ion concentration of $1.2×10^{−3}\;m$?.

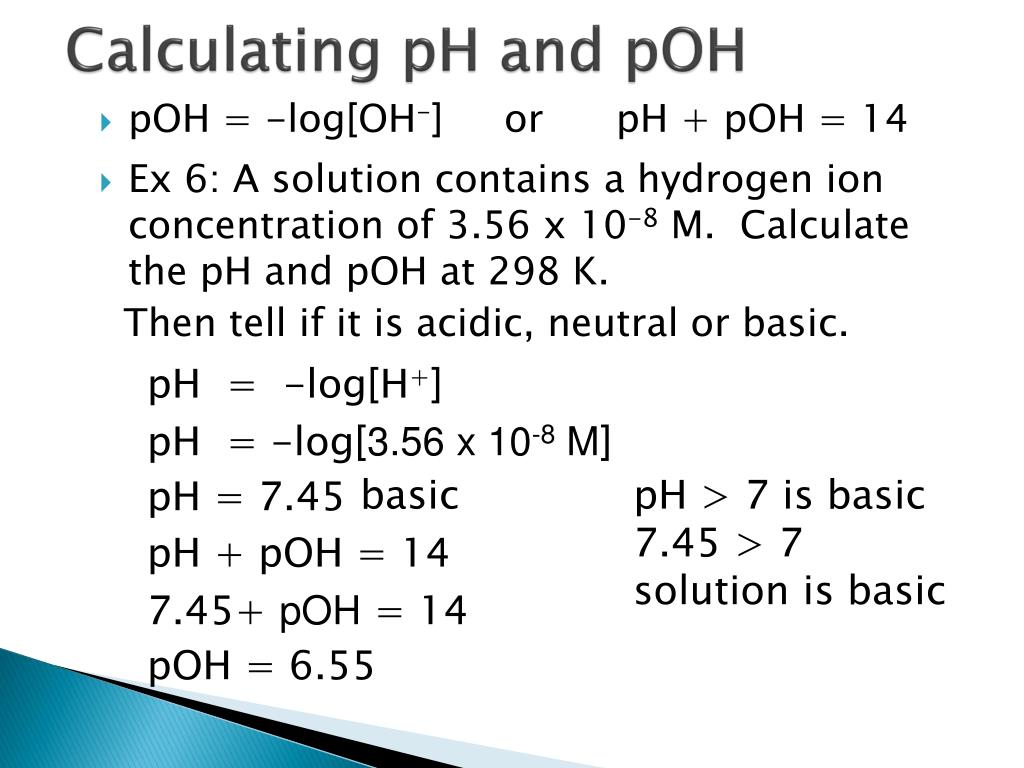
Calculation Of Ph And Poh Calculating ph. to calculate the ph of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter (molarity). the ph is then calculated using the expression: ph = log [h 3 o ]. example: find the ph of a 0.0025 m hcl solution. the hcl is a strong acid and is 100% ionized in water. Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol l. calculate ph by using the ph to h⁺ formula: \qquad \small\rm ph = log (0.0001) = 4 ph = −log(0.0001) = 4. now, you can also easily determine poh and a concentration of hydroxide ions using the formulas:. In terms of hydronium ion concentration, the equation to determine the ph of an aqueous solution is: \[ph = \log[h {3}o^ ]\] poh: the poh of an aqueous solution, which is related to the ph, can be determined by the following equation: \[poh = \log[oh^ ]\] this equation uses the hydroxide concentration of an aqueous solution instead of the. Calculate [h 3 o ], [oh −], ph, and poh for pure water at 40 °c. the ionization constant for water (k w) is 9.614 × 10 −14 at 60 °c. calculate [h 3 o ], [oh −], ph, and poh for pure water at 60 °c. calculate the ph and the poh of each of the following solutions at 25 °c for which the substances ionize completely: 0.200 m hcl; 0.

Comments are closed.