Ph And Poh Scale

What Is Poh Definition And Calculation Template:openstax. 14.9: the ph and poh scales ways to express acidity and basicity is shared under a license and was authored, remixed, and or curated by libretexts. ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. knowledge of ether can be used to calculate either [h ] of [oh. It is likely you have only heard of the ph scale. however, there is a ph counterpart called the poh (the "power of the hydroxide ion"), which is defined as the negative logarithm of the hydroxide ion concentration: \[\mathrm{poh}= \log\left[\mathrm{oh}^ \right]\] for aqueous solutions at 25°c, the sum of the ph and poh is always 14.00:.
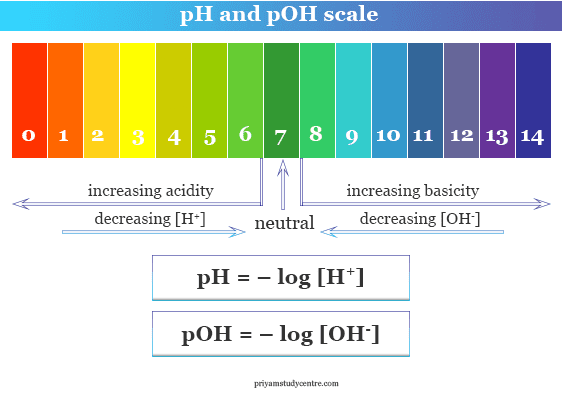
Ph Scale Poh Scale Definition Range Chart Measurement Learn how to define and use ph and poh scales to describe the acidity or basicity of aqueous solutions. see examples, typical ph values of common substances, and how to calculate [h ] and [oh ] from ph and poh. By definition, poh is the negative logarithm (to the base 10) of the hydroxide ion concentration (mol l). poh values can be derived from ph measurements and vice versa. the concentration of hydroxide ions in water is related to the concentration of hydrogen ions by. where kw is the self ionization constant of water. If you're seeing this message, it means we're having trouble loading external resources on our website. if you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Learn what poh is and how to find it from hydroxide ion concentration or ph. see the poh scale and how it relates to ph for acids and bases.
.PNG)
Ph And Poh Calculations If you're seeing this message, it means we're having trouble loading external resources on our website. if you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Learn what poh is and how to find it from hydroxide ion concentration or ph. see the poh scale and how it relates to ph for acids and bases. The product of hydrogen ion, hydroxyl has a constant that has a value of 10 14. thus, from the definition of sørensen, it follows that ph poh = 14, and hence poh = 14 – ph. so this ph scale varies from 0 to 14. ph of dissolution of pure water. examples of measure values on the ph scale. for sulfuric acid (h 2 so 4) 0.01 m:. Learn what ph and poh are, how to convert them, and how they relate to acidity and alkalinity. find the ph and poh values of some common molecules and the difference between them.

Comments are closed.