Phase Diagram Of Water Vs Co2

Phase Diagrams Of A Water And B Carbon Dioxide Co 2 The February 7, 2024. by hasini a. 6 min read. the main difference between phase diagram of water and carbon dioxide is the phase diagram of water includes a distinct liquid phase under atmospheric pressure, while carbon dioxide sublimes directly from solid to gas at similar pressures. a phase diagram is a graphical representation that illustrates. This chemistry video tutorial explains the concepts behind the phase diagram of co2 carbon dioxide and the phase diagram of water h2o. this video contai.

Phase Diagram Of Water Vs Other Substances Differences Meaning Example 12.4.1 12.4. 1: water. referring to the phase diagram of water in figure 12.4.2 12.4. 2: predict the physical form of a sample of water at 400°c and 150 atm. describe the changes that occur as the sample in part (a) is slowly allowed to cool to −50°c at a constant pressure of 150 atm. Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram. under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. at 2, it would be a liquid; and at 3, it would be a vapor (a gas). A phase diagram identifies the physical state of a substance under specified conditions of pressure and temperature. to illustrate the utility of these plots, consider the phase diagram of water, shown below. figure 2. phase diagram of water. a pressure of 50 kpa and a temperature of −10 °c corresponds to the region of the diagram labeled. A phase diagram identifies the physical state of a substance under specified conditions of pressure and temperature. to illustrate the utility of these plots, consider the phase diagram of water, shown below. figure 2. phase diagram of water. a pressure of 50 kpa and a temperature of −10 °c corresponds to the region of the diagram labeled.
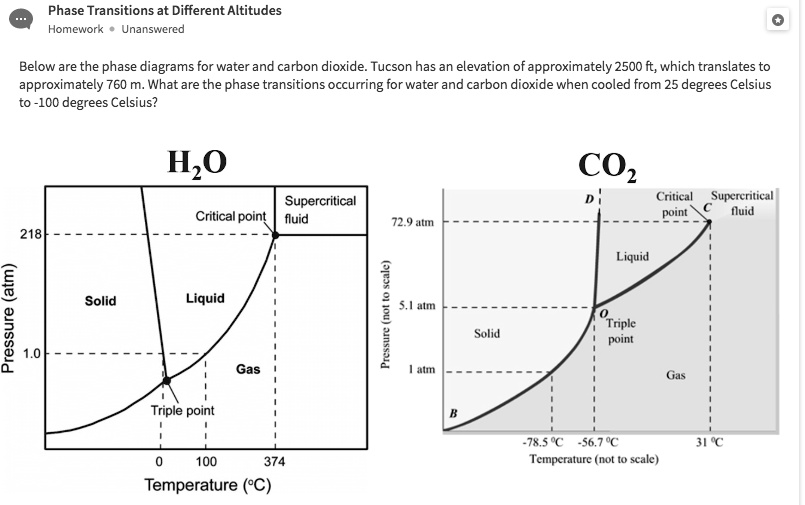
Solved Below Are The Phase Diagrams For Water And Carbon Dioxide A phase diagram identifies the physical state of a substance under specified conditions of pressure and temperature. to illustrate the utility of these plots, consider the phase diagram of water, shown below. figure 2. phase diagram of water. a pressure of 50 kpa and a temperature of −10 °c corresponds to the region of the diagram labeled. A phase diagram identifies the physical state of a substance under specified conditions of pressure and temperature. to illustrate the utility of these plots, consider the phase diagram of water, shown below. figure 2. phase diagram of water. a pressure of 50 kpa and a temperature of −10 °c corresponds to the region of the diagram labeled. The phase diagram of carbon dioxide (co 2) is shown below. it has a melting curve (bd), vaporization curve (bc), and sublimation curve (ab). unlike water, the melting curve of carbon dioxide slopes towards the right. point b is known as the triple point. it represents the pressure and temperature at which all three phases of a substance can. Determining the state of water using the phase diagram for water given in figure 10.31, determine the state of water at the following temperatures and pressures: (a) −10 °c and 50 kpa (b) 25 °c and 90 kpa (c) 50 °c and 40 kpa (d) 80 °c and 5 kpa (e) −10 °c and 0.3 kpa (f) 50 °c and 0.3 kpa. solution.

Phase Diagram Of Water Vs Co2 The phase diagram of carbon dioxide (co 2) is shown below. it has a melting curve (bd), vaporization curve (bc), and sublimation curve (ab). unlike water, the melting curve of carbon dioxide slopes towards the right. point b is known as the triple point. it represents the pressure and temperature at which all three phases of a substance can. Determining the state of water using the phase diagram for water given in figure 10.31, determine the state of water at the following temperatures and pressures: (a) −10 °c and 50 kpa (b) 25 °c and 90 kpa (c) 50 °c and 40 kpa (d) 80 °c and 5 kpa (e) −10 °c and 0.3 kpa (f) 50 °c and 0.3 kpa. solution.

Comments are closed.