Phase Diagrams Of Water Co2 Explained Chemistry Melting Boiling Critical Point
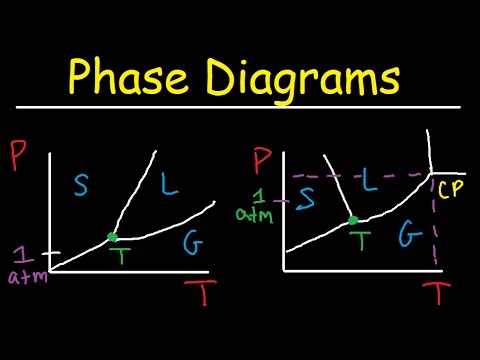
Phase Diagrams Of Water Co2 Explained Chemistry Melting ођ This chemistry video tutorial explains the concepts behind the phase diagram of co2 carbon dioxide and the phase diagram of water h2o. this video contai. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. in figure 12.4.1 12.4. 1, the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with pressure. the solid and liquid phases are in.

Phase Diagram Of Water And Carbon Dioxide Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram. under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. at 2, it would be a liquid; and at 3, it would be a vapor (a gas). 10:28. phase diagrams of water & co2 explained chemistry melting, boiling & critical point. the organic chemistry tutor. Imagine a substance with the following points on the phase diagram: a triple point at .5 atm and 5°c; a normal melting point at 20°c; a normal boiling point at 150°c; and a critical point at 5 atm and 1000°c. the solid liquid line is "normal" (meaning positive sloping). for this, complete the following: 1. Consider the phase diagram for carbon dioxide shown in figure 10.34 as another example. the solid liquid curve exhibits a positive slope, indicating that the melting point for co 2 increases with pressure as it does for most substances (water being a notable exception as described previously).

Carbon Dioxide Co2 Phase Diagram Imagine a substance with the following points on the phase diagram: a triple point at .5 atm and 5°c; a normal melting point at 20°c; a normal boiling point at 150°c; and a critical point at 5 atm and 1000°c. the solid liquid line is "normal" (meaning positive sloping). for this, complete the following: 1. Consider the phase diagram for carbon dioxide shown in figure 10.34 as another example. the solid liquid curve exhibits a positive slope, indicating that the melting point for co 2 increases with pressure as it does for most substances (water being a notable exception as described previously). We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. for example, a pressure of 50 kpa and a temperature of −10 °c correspond to the region of the diagram labeled “ice.”. under these conditions, water exists only as a solid (ice). A phase diagram identifies the physical state of a substance under specified conditions of pressure and temperature. to illustrate the utility of these plots, consider the phase diagram of water, shown below. figure 2. phase diagram of water. a pressure of 50 kpa and a temperature of −10 °c corresponds to the region of the diagram labeled.

Phase Change Diagram Of Water вђ Overview Importance Expii We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. for example, a pressure of 50 kpa and a temperature of −10 °c correspond to the region of the diagram labeled “ice.”. under these conditions, water exists only as a solid (ice). A phase diagram identifies the physical state of a substance under specified conditions of pressure and temperature. to illustrate the utility of these plots, consider the phase diagram of water, shown below. figure 2. phase diagram of water. a pressure of 50 kpa and a temperature of −10 °c corresponds to the region of the diagram labeled.

Comments are closed.